

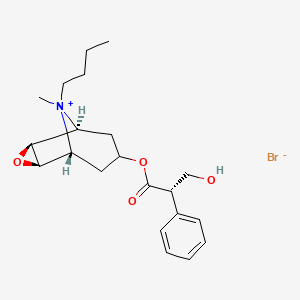

1. Bromide, Butylscopolammonium
2. Bromide, N-butylscopolammonium
3. Buscapine
4. Buscolysin
5. Buscopan
6. Butylscopolamine
7. Butylscopolammonium Bromide
8. Hyoscinbutylbromide
9. Hyoscine N Butylbromide
10. Hyoscine N-butylbromide
11. N Butylscopolammonium Bromide
12. N-butylbromide, Hyoscine
13. N-butylscopolammonium Bromide
14. Scopolaminebutylbromide
15. Scopolan
1. 149-64-4
2. N-butylscopolammonium Bromide
3. Hyoscine Butylbromide
4. Buscopan
5. Scopolan
6. Buscapine
7. Buscolysin
8. Scobutil
9. Sporamin
10. Amisepan
11. Buscapina
12. Butylmin
13. Donopon
14. Joscine
15. Monospan
16. Scobron
17. Scobutyl
18. Sparicon
19. Tirantil
20. Buscol
21. Scobro
22. Buscolamin
23. Butylscopolammonium Bromide
24. Butylscopolamine Bromide
25. Scopolamine Bromobutylate
26. Hyoscine-n-butyl Bromide
27. Buscolysine
28. Stilbron
29. Hyoscine Butyl Bromide
30. N-butylhyoscinium Bromide
31. Butylhyoscine
32. Scopolamine Butobromide
33. N-butylhyoscine Bromide
34. Scoburen
35. Stibron
36. Hyoscin-n-butyl Bromide
37. Scopolamine Butyl Bromide
38. Scopolamine N-butylbromide
39. N-butylscopolamine Bromide
40. Scopolamine N-butyl Bromide
41. N-butylscopolaminium Bromide
42. (-)-n-butylscopolamine Bromide
43. (-)-scopolamine Butylbromide
44. Mls000069755
45. 0gh9jx37c8
46. (-)-scopolamine N-butyl Bromide
47. Smr000058795
48. Dsstox_cid_2718
49. Dsstox_rid_76697
50. Dsstox_gsid_22718
51. Cas-149-64-4
52. Hyoscine Butobromide
53. Hyoscin-n-butylbromid
54. Butylscopolamin
55. Unii-0gh9jx37c8
56. Buskolamin
57. Antipan
58. Buscogast
59. Hyocimax
60. Scopinal
61. Spasmin
62. Hybrocare
63. Butylscopolamine Bromide [jan]
64. Sr-01000759230
65. Hyoscin-n-butylbromid [german]
66. Spasler-p
67. Hyoscin Butobromide
68. (1?,2?,4?,5?,7?)-9-butyl-7-[(2s)-3-hydroxy-1-oxo-2-phenylpropoxy]-9-methyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonane Bromide
69. Einecs 205-744-1
70. Mfcd00078561
71. Opera_id_556
72. Ncgc00163224-01
73. Scopolamine (butylbromide)
74. Scopolamine N-n-butylbromide
75. Hyoscine-n-butyl Bromide Bp
76. Schembl25291
77. Mls002153372
78. Mls006013629
79. Chembl1256901
80. Dtxsid1022718
81. Chebi:32123
82. Hms2235k20
83. Hms3714i19
84. Hms3884j15
85. Hy-n0340
86. Tox21_112031
87. Hyoscine Butylbromide [mart.]
88. Scopolamine Butylbromide [jan]
89. Akos016009548
90. Akos037515799
91. Hyoscine Butylbromide [who-dd]
92. Tox21_112031_1
93. Ccg-208412
94. Cs-3142
95. Ncgc00186628-02
96. 3-oxa-9-azoniatricyclo(3.3.1.02,4)nonane, 9-butyl-7-(3-hydroxy-1-oxo-2-phenylpropoxy)-9-methyl-, Bromide, (7(s)-(1alpha,2beta,4beta,5alpha,7beta))-
97. Ac-34139
98. As-78017
99. N-butylscopolammonium Bromide [mi]
100. Smr004705104
101. Hyoscine Butylbromide [ep Monograph]
102. Butylscopolamine Bromide [green Book]
103. F17660
104. A884250
105. J-008603
106. Sr-01000759230-3
107. N-butyl Scopolamine Bromide (n-butyl Hyoscine Bromide)
108. (-)-scopolamine N-butyl Bromide, >=98% (tlc), Powder
109. Hyoscine Butylbromide, British Pharmacopoeia (bp) Reference Standard
110. Hyoscine Butylbromide, European Pharmacopoeia (ep) Reference Standard
111. (1r,2r,4s,5s,7s)-9-butyl-7-{[(2s)-3-hydroxy-2-phenylpropanoyl]oxy}-9-methyl-3-oxa-9-azoniatricyclo[3.3.1.0~2,4~]nonane Bromide
112. (2r,4s,5s,7s)-9-butyl-7-{[(2s)-3-hydroxy-2-phenylpropanoyl]oxy}-9-methyl-3-oxa-9-azoniatricyclo[3.3.1.0(2,4)]nonane Bromide
113. [(1s,2s,4r,5r)-9-butyl-9-methyl-3-oxa-9-azoniatricyclo[3.3.1.02,4]nonan-7-yl] (2s)-3-hydroxy-2-phenylpropanoate;bromide
114. 1-alpha-h,5-alpha-h-tropanium, 8-butyl-6-beta,7-beta-epoxy-3-alpha-hydroxy-, Bromide, (-)-tropate
115. 3-oxa-9-azoniatricyclo(3.3.1.0 Sup(2,4))nonane, 9-butyl-7-((2s)-3-hydroxy-1-oxo-2-phenylpropoxy)-9-methyl-, Bromide (1:1),(1.alpha.,2.beta.,4.beta.,5.alpha.,7.beta.)-
| Molecular Weight | 440.4 g/mol |
|---|---|
| Molecular Formula | C21H30BrNO4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 439.13582 g/mol |
| Monoisotopic Mass | 439.13582 g/mol |
| Topological Polar Surface Area | 59.1 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 500 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
