

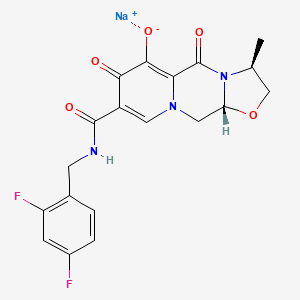

1. (3s,11ar)-n-((2,6-difluoropyridin-3-yl)methyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro(1,3)oxazolo(3,2-a)pyrido(1,2-d)pyrazine-8-carboxamide
2. Apretude
3. Cabotegravir
4. Cabotegravir Extended-release Injectable Suspension
5. Gsk-1265744
6. Gsk-1265744a
7. Gsk-1265744b
8. Gsk1265744
9. Gsk1265744a
10. Gsk1265744b
11. Gsk744
12. N-((2,4-difluorophenyl)methyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro(1,3)oxazolo(3,2-a)pyrido(1,2-d)pyrazine-8-carboxamide
13. S-265744
14. S-265744b
15. Sodium (3s,11ar)-8-(((2,4-difluorophenyl)methyl)carbamoyl)-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydrooxazolo(3,2-a)pyrido(1,2-d)pyrazin-6-olate
16. Vocabria
1. 1051375-13-3
2. Gsk1265744b
3. Gsk-1265744b
4. Vocabria
5. S-265744b
6. Cabotegravir Sodium [usan]
7. Cabotegravir (sodium)
8. S 265744b
9. 3l12pt535m
10. Sodium (3s,11ar)-8-((2,4-difluorobenzyl)carbamoyl)-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydrooxazolo[3,2-a]pyrido[1,2-d]pyrazin-6-olate
11. Oxazolo(3,2-a)pyrido(1,2-d)pyrazine-8-carboxamide, N-((2,4-difluorophenyl)methyl)-2,3,5,7,11,11a-hexahydro-6-hydroxy-3-methyl-5,7-dioxo-, Sodium Salt (1:1), (3s,11ar)-
12. Sodium (3s,11ar)-8-(((2,4-difluorophenyl)methyl)carbamoyl)-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydrooxazolo(3,2-a)pyrido(1,2-d)pyrazin-6-olate
13. Unii-3l12pt535m
14. Chembl3137330
15. Cabotegravir Sodium (jan/usan)
16. Cabotegravir Sodium [jan]
17. Chebi:172948
18. Amy30044
19. Cabotegravir Sodium [who-dd]
20. Gsk 1265744b
21. Hy-15592a
22. Cabotegravir Sodium [orange Book]
23. Ac-30853
24. Cs-0007799
25. D10549
26. F11513
27. A935003
28. Q27257476
29. (3s,11ar)-n-(2,4-difluorobenzyl)-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydrooxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide, Sodium Salt
30. 1229006-16-9
31. Sodium (3s,11ar)-8-[(2,4-difluorobenzyl)carbamoyl]-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazin-6-olate
32. Sodium(3s,11ar)-8-((2,4-difluorobenzyl)carbamoyl)-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydrooxazolo[3,2-a]pyrido[1,2-d]pyrazin-6-olate
| Molecular Weight | 427.3 g/mol |
|---|---|
| Molecular Formula | C19H16F2N3NaO5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 427.09557123 g/mol |
| Monoisotopic Mass | 427.09557123 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 820 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Vocabria tablets are indicated in combination with rilpivirine tablets for the short-term treatment of Human Immunodeficiency Virus type 1 (HIV-1) infection in adults who are virologically suppressed (HIV-1 RNA < 50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with agents of the NNRTI and INI class for:
- oral lead in to assess tolerability of Vocabria and rilpivirine prior to administration of long acting cabotegravir injection plus long acting rilpivirine injection.
- oral therapy for adults who will miss planned dosing with cabotegravir injection plus rilpivirine injection.
Vocabria injection is indicated, in combination with rilpivirine injection, for the treatment of Human Immunodeficiency Virus type 1 (HIV-1) infection in adults who are virologically suppressed (HIV-1 RNA < 50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with agents of the NNRTI and INI class.
HIV Integrase Inhibitors
Inhibitors of HIV INTEGRASE, an enzyme required for integration of viral DNA into cellular DNA. (See all compounds classified as HIV Integrase Inhibitors.)
J05AX

