


1. Camostat Mesilate
2. Camostat Mesylate
3. Camostate
4. Camostate-mesilate
5. Foipan
6. Foy 305
7. Foy S 980
8. Foy-305
9. Foypan
10. N,n-dimethylcarbamoylmethyl-4-(4-guanidinobenzoyloxy)phenylacetate Methanesulfonate
11. P-guanidinobenzoic Acid, Ester With (p-hydroxyphenyl)acetic Acid, Ester With N,n-dimethylglycolamide
1. 59721-28-7
2. Camostat [inn]
3. Camostate
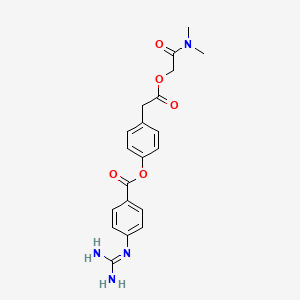
4. [4-[2-[2-(dimethylamino)-2-oxoethoxy]-2-oxoethyl]phenyl] 4-(diaminomethylideneamino)benzoate
5. 0fd207wkdu
6. 4-(2-(2-(dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl 4-guanidinobenzoate
7. Camostat (inn)
8. Foipan
9. P-guanidinobenzoic Acid, Ester With (p-hydroxyphenyl)acetic Acid, Ester With N,n-dimethylglycolamide
10. Camostatum
11. Camostatum [inn-latin]
12. Chembl85164
13. Ccris 7219
14. Ncgc00167526-01
15. Unii-0fd207wkdu
16. Camostat-mesilate
17. Dimethylcarbamoylmethyl 4-(4-guqnidinobenzoyloxy)phenylacetat
18. Camostat [mi]
19. Camostat [who-dd]
20. Us9199927, Camostat
21. Schembl125269
22. Chembl590799
23. Gtpl6432
24. Dtxsid6044010
25. Chebi:135632
26. Bdbm193418
27. Bcp22042
28. Ex-a5738
29. Zinc3871842
30. Bdbm50031706
31. Bdbm50424712
32. Bcp9000475
33. Db13729
34. Ncgc00167526-03
35. Db-053447
36. Ft-0630715
37. D07606
38. 721c287
39. Q5026909
40. 2-(dimethylamino)-2-oxoethyl4-(4-guanidinobenzoyloxy)phenylacetate
41. 4-(2-(2-(dimethylamino)-2-oxoethoxy)-2-oxoethyl)phenyl4-guanidinobenzoate
42. N,n-dimethyl Carbamoylmethyl-p-(p-guanidinobenzoyloxy) Phenyl-acetate
43. [4-[2-(2-dimethylamino-2-oxoethoxy)-2-oxoethyl]phenyl] 4-(diaminomethylideneamino)benzoate
44. 4-{2-[(dimethylcarbamoyl)methoxy]-2-oxoethyl}phenyl 4-carbamimidamidobenzoate
45. 4-guanidino-benzoic Acid 4-dimethylcarbamoylmethoxycarbonylmethyl-phenyl Ester; Compound With Methanesulfonic Acid
46. Benzeneacetic Acid,4-((4-((aminoiminomethyl)amino)benzoyl)oxy)-,2-(dimethylamino)-2-oxoethyl Ester
| Molecular Weight | 398.4 g/mol |
|---|---|
| Molecular Formula | C20H22N4O5 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 398.15901982 g/mol |
| Monoisotopic Mass | 398.15901982 g/mol |
| Topological Polar Surface Area | 137 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 602 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Camostat mesylate is indicated in Japan to treat chronic pancreatitis and drug induced lung injury. It is also being investigated as a potential treatment for COVID-19.
Camostat mesylate is a protease inhibitor used to treat chronic pancreatitis. The duration of action is not long, as it is typically given in 3 divided doses daily. Patients should be counselled regarding the risk of anaphylaxis, thrombocytopenia, hepatic dysfunction, and hyperkalemia.
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
Trypsin Inhibitors
Serine proteinase inhibitors which inhibit trypsin. They may be endogenous or exogenous compounds. (See all compounds classified as Trypsin Inhibitors.)
B - Blood and blood forming organs
B02 - Antihemorrhagics
B02A - Antifibrinolytics
B02AB - Proteinase inhibitors
B02AB04 - Camostat
Absorption
A 200mg oral dose of camostat mesylate leads to the active metabolite reaching a Cmax of 87.1 29.5 ng/mL, with a Tmax of 40 min, and an AUC of 10,400 1,400 ng\*min/mL.
Route of Elimination
Camostat mesylate is 89.8-95.6% eliminated in the urine and 1.0-1.7% eliminated in the feces.
Volume of Distribution
The volume of distribution at steady state of camostat mesylate is 0.34-1.31L/kg.
Clearance
The clearance of camostat mesylate is 4.5-7.3mL/min/kg.
Camostat mesylate is hydrolyzed by carboxyesterate to the active 4-(4-guanidinobenzoyloxy) phenylacetate. The active metabolite is further hydrolyzed by arylesterase to 4-guanidinobenzoic acid.
The half life of camostat mesylate is 3.8-4.7h.
In rats, oral camostat mesylate may increase pancreatic secretions and hypertrophy by increasing cholecystokinin release. Administration in rats has also lead to lower levels of IL-1beta, IL-6, TNF-alpha, TGF-beta, and PSC. Similar activity is seem after administration in humans, leading to reduced pain and inflammation as well as improve the function of the pancrease in chronic pancreatitis. In the case of SARS-CoV-2, camostat mesylate inhibits the action of the serine protease TMPRSS2, preventing the priming of the viral spike protein for attachment to ACE2, and entry into the cell.
