

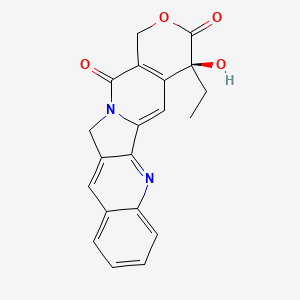

1. Camptothecine
1. Camptothecine
2. 7689-03-4
3. (s)-(+)-camptothecin
4. Campathecin
5. (+)-camptothecine
6. D-camptothecin
7. (+)-camptothecin
8. 20(s)-camptothecine
9. 21,22-secocamptothecin-21-oic Acid Lactone
10. Nsc94600
11. Camptothecine (s,+)
12. Chembl65
13. (4s)-4-ethyl-4-hydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
14. Nsc-94600
15. Topotecan Related Compound C
16. (s)-4-ethyl-4-hydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
17. Mls000766223
18. Xt3z54z28a
19. Chebi:27656
20. Mfcd00081076
21. (19s)-19-ethyl-19-hydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4,6,8,10,15(20)-heptaene-14,18-dione
22. Nsc 100880
23. 251316-95-7
24. (s)-camptothecin
25. (19s)-19-ethyl-19-hydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.0^{2,11}.0^{4,9}.0^{15,20}]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione
26. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-, (4s)-
27. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-, (s)-
28. 1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-, (4s)-
29. 20(s)-camptothecin
30. 4-ethyl-4-hydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
31. Sr-01000075798
32. Sr-01000597379
33. D-camptothecine
34. (s)-camptothecine
35. Camptothecin,(s)
36. ( )-camptothecin
37. (4s)-4-ethyl-4-hydroxy-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione
38. (s)-4-ethyl-4-hydroxy-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione
39. (s)-4-ethyl-4-hydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-(4h,12h)-dione
40. 1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-, (s)-
41. Prestwick_102
42. (+)-camptothecin;
43. Camptothecine (8ci)
44. Spectrum_000299
45. Tocris-1100
46. Specplus_000712
47. Prestwick0_000200
48. Prestwick1_000200
49. Prestwick2_000200
50. Prestwick3_000200
51. Spectrum2_000903
52. Spectrum3_001203
53. Spectrum4_000738
54. Spectrum5_001126
55. Camptothecin [mi]
56. Lopac-c-9911
57. Schembl6038
58. Unii-xt3z54z28a
59. Lopac0_000341
60. Bspbio_000159
61. Bspbio_002586
62. Kbiogr_001036
63. Kbioss_000779
64. Kbioss_002283
65. Cid_24360
66. Camptothecin [who-dd]
67. Divk1c_000826
68. Divk1c_006808
69. Spectrum1502232
70. Spbio_000746
71. Spbio_002080
72. Bpbio1_000175
73. Ccris 8162
74. Dtxsid0030956
75. Hms502j08
76. Kbio1_000826
77. Kbio1_001752
78. Kbio2_000779
79. Kbio2_003347
80. Kbio2_005915
81. Kbio3_002086
82. 4-ethyl-4-hydroxy-1h-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
83. Ninds_000826
84. Bio1_000400
85. Bio1_000889
86. Bio1_001378
87. Hms1568h21
88. Hms1921n08
89. Hms2089f08
90. Hms2095h21
91. Hms3261e03
92. Hms3414j17
93. Hms3654d13
94. Hms3678j15
95. Hms3712h21
96. Zinc105309
97. Act02668
98. Bcp02857
99. Tox21_500341
100. Ac-202
101. Bbl033963
102. Bdbm50008923
103. Ccg-40255
104. Gr-301
105. Nsc 94600
106. S1288
107. Stk801886
108. Akos004119861
109. Cs-1049
110. Db04690
111. Ks-5235
112. Lp00341
113. Sdccgmls-0066688.p001
114. Sdccgsbi-0050329.p003
115. Brn 0631069
116. Cas-2114454
117. Idi1_000826
118. Ncgc00015290-01
119. Ncgc00016994-01
120. Ncgc00016994-02
121. Ncgc00016994-03
122. Ncgc00016994-04
123. Ncgc00016994-05
124. Ncgc00016994-06
125. Ncgc00016994-07
126. Ncgc00016994-08
127. Ncgc00016994-09
128. Ncgc00016994-10
129. Ncgc00016994-11
130. Ncgc00016994-12
131. Ncgc00016994-16
132. Ncgc00016994-29
133. Ncgc00024997-01
134. Ncgc00024997-02
135. Ncgc00024997-03
136. Ncgc00024997-04
137. Ncgc00024997-05
138. Ncgc00024997-06
139. Ncgc00178592-01
140. Ncgc00178592-02
141. Ncgc00261026-01
142. Hy-16560
143. Nci60_042105
144. Smr000445686
145. Sy010324
146. Ai3-62475
147. Eu-0100341
148. N1868
149. Sw196414-3
150. Topotecan Related Compound C [usp-rs]
151. C 9911
152. C01897
153. M01564
154. Ab00052452-08
155. Ab00052452-09
156. Ab00052452_10
157. (s)-(+)-camptothecin, >=90% (hplc), Powder
158. 689c034
159. A838882
160. Q419964
161. Q-200785
162. Sr-01000075798-1
163. Sr-01000075798-4
164. Sr-01000597379-1
165. Sr-01000597379-3
166. Brd-k37890730-001-09-4
167. Brd-k37890730-001-10-2
168. (s)-4-ethyl-4-hydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]florene-3,13-dione
169. (s)-4-ethyl-4-hydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
170. 4-ethyl-4-hydroxy-1h-pyrano-[3[,4[:6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
171. Topotecan Related Compound C, United States Pharmacopeia (usp) Reference Standard
172. (s)-4-ethyl-4-hydroxy-1h-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
173. (s)-4-ethyl-4-hydroxy-1h-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione;(s)-(+)-camptothecin
174. (s)-4-ethyl-4-hydroxy-1h-pyrano[3 Inverted Exclamation Mark ,4 Inverted Exclamation Mark :6,7]indolizino[1,2-b]quinoline-3,14-(4h,12h)-dione
175. 1h-pyrano[3',7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione, 4-ethyl-4-hydroxy-, (s)-
176. 4(s)-ethyl-4-hydroxy-1h-pyrano-[3',4':6,7]indolizino[1,2-b]quinoline-3,14 (4h,12h)-dione
177. 4-ethyl-4-hydroxy-(4s)-3,4,12,14-tetrahydro-1h-pyrano[3'',4'':6,7]indolizino[1,2-b]quinoline-3,14-dione
178. 4-ethyl-4-hydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (camptothecin Or Cpt)
179. 4-ethyl-4-hydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (camptothecin)
180. 4-ethyl-4-hydroxy-1,12-dihydro-4h-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (cpt, Camptothecin)
| Molecular Weight | 348.4 g/mol |
|---|---|
| Molecular Formula | C20H16N2O4 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 348.11100700 g/mol |
| Monoisotopic Mass | 348.11100700 g/mol |
| Topological Polar Surface Area | 79.7 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 742 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for the treatment of cancer.
Camptothecin demonstrated strong anticancer activity in preliminary clinical trials but also low solubility and adverse drug reaction. Camptothecin is believed to be a potent topoisomerase inhibitor that interferes with the essential function of topoisomerase in DNA replication.
Topoisomerase I Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE I. (See all compounds classified as Topoisomerase I Inhibitors.)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Camptothecin binds to the topoisomerase I and DNA complex resulting in a ternary complex, stabilizing it and preventing DNA re-ligation and therefore causes DNA damage which results in apoptosis.