
1. Ci 1033
2. Ci-1033
3. Ci1033
1. 267243-28-7
2. Ci-1033
3. Pd-183805
4. Canertinib (ci-1033)
5. Canertinib Free Base
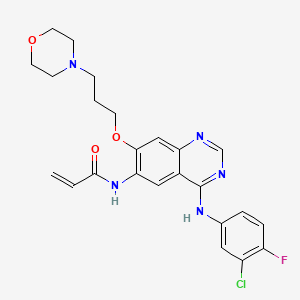
6. N-(4-(3-chloro-4-fluorophenylamino)-7-(3-morpholinopropoxy)quinazolin-6-yl)acrylamide
7. N-{4-[(3-chloro-4-fluorophenyl)amino]-7-[3-(morpholin-4-yl)propoxy]quinazolin-6-yl}prop-2-enamide
8. C78w1k5asf
9. Chembl31965
10. N-[4-(3-chloro-4-fluoroanilino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]prop-2-enamide
11. Chebi:61399
12. 267243-28-7 (free Base)
13. N-(4-((3-chloro-4-fluorophenyl)amino)-7-(3-(morpholin-4-yl)propoxy)quinazolin-6-yl)prop-2-enamide
14. Ncgc00182713-01
15. Pd183805
16. Canertinib [inn]
17. Canertinib [inn:ban]
18. Sn-26606
19. Ci-1033(canertinib)
20. Ci1033
21. Unii-c78w1k5asf
22. Canetinib
23. Canertinib [mi]
24. Caneritinib; Ci-1033
25. Canertinib - Ci-1033
26. Ci-1033 (canertinib)
27. Canertinib [who-dd]
28. Dsstox_cid_28869
29. Dsstox_rid_83138
30. Dsstox_gsid_48943
31. Schembl54837
32. Mls004774146
33. Pd 183805 Dihydrochloride
34. Bdbm4779
35. Cid_156414
36. Gtpl5675
37. Dtxsid8048943
38. Ex-a078
39. Bcpp000301
40. Hms3244m17
41. Hms3244m18
42. Hms3244n17
43. Bcp01790
44. Tox21_113361
45. Nsc780019
46. Nsc801011
47. S1019
48. Zinc27439698
49. Akos005145818
50. Bcp9000481
51. Bcp9000482
52. Bcp9000525
53. Ccg-269588
54. Cs-0121
55. Db05424
56. Nsc-780019
57. Nsc-801011
58. Sb16594
59. N-(4-((3-chloro-4-fluorophenyl)amino)-7-(3-morpholinopropoxy)quinazolin-6-yl)acrylamide
60. N-[4-(3-chloro-4-fluoro-anilino)-7-(3-morpholinopropoxy)quinazolin-6-yl]prop-2-enamide
61. Ncgc00182713-02
62. Ncgc00182713-18
63. As-56189
64. Hy-10367
65. Smr003500789
66. Pd0183805
67. Cas-267243-28-7
68. Ft-0654215
69. Ec-000.2258
70. A25038
71. 499c452
72. Q5032274
73. 2-propenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-(3-(4-morpholinyl)propoxy)-6-quinazolinyl)-
74. N-(4-(3-chloro-4-fluorophenyl)amino)-7-(3-morpholin-4-yl)propoxy)quinazolin-6-yl)prop-2-enamide
75. N-[4-(3-chloro-4-fluoro-phenyl-amino)-7-(3-morpholin-4-yl-propoxy)-quinazolin-6-yl]-acrylamide
| Molecular Weight | 485.9 g/mol |
|---|---|
| Molecular Formula | C24H25ClFN5O3 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 485.1629955 g/mol |
| Monoisotopic Mass | 485.1629955 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 671 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in breast cancer and lung cancer.
CI-1033 effectively inhibits the growth of esophageal squamous cell carcinoma which co-expresses both EGFR and HER2 with the inhibition of phosphorylation of both MAPK and AKT. Some studies suggest that CI-1033 holds significant clinical potential in esophageal cancer.
