
1. C.i. 40850
2. Canthaxanthine
3. Carophyll Red
4. Food Orange 8
5. Orobronze
6. Roxanthin Red 10
1. 514-78-3
2. Orobronze
3. Carophyll Red
4. Beta,beta-carotene-4,4'-dione
5. Food Orange 8
6. Cantaxanthin
7. Canthaxanthine
8. Roxanthin Red 10
9. Cantaxanthine
10. L-orange 7
11. 4,4'-dioxo-beta-carotene
12. Canthaxanthin (trans)
13. Canthaxanthin, Powder
14. Ci 40850
15. All-trans,beta-carotene-4,4'-dione
16. Chebi:3362
17. 4c3c6403mu
18. Nsc-374110
19. Ncgc00095896-01
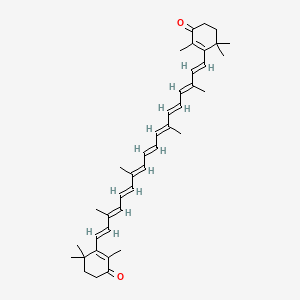
20. 2,4,4-trimethyl-3-[(1e,3e,5e,7e,9e,11e,13e,15e,17e)-3,7,12,16-tetramethyl-18-(2,6,6-trimethyl-3-oxocyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohex-2-en-1-one
21. C.i. Food Orange 8
22. All-trans-canthaxanthin
23. 3,3'-((1e,3e,5e,7e,9e,11e,13e,15e,17e)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,4,4-trimethylcyclohex-2-en-1-one)
24. Carotene-4,4'-dione, Beta-
25. Ccris 3276
26. E 161g
27. Einecs 208-187-2
28. Nsc 374110
29. Ro 1-9915
30. E 161 G
31. Brn 1898520
32. Beta-carotene-4,4'-dione, All-trans-
33. Unii-4c3c6403mu
34. Canthaxanthin (euglenanone)
35. Mfcd00016364
36. Cantha
37. Kantakisantin
38. Canthaxanthin, Tech.
39. Lucantin Red
40. Isomer Of Canthaxanthin
41. 4,4'-diketo-b-carotene
42. Ci-food Orange 8
43. Dsstox_cid_2727
44. Canthaxanthin [mi]
45. Beta-carotin-4,4?-dione
46. Canthaxanthin [fcc]
47. 4,4'-diketo-beta-carotene
48. Dsstox_rid_76702
49. Dsstox_gsid_22727
50. Schembl19618
51. 4-07-00-02680 (beilstein Handbook Reference)
52. Canthaxanthin [mart.]
53. E161g
54. Ins No.161g
55. Spectrum1504204
56. Canthaxanthin [who-dd]
57. Ins-161g
58. Chembl1329004
59. Dtxsid0022727
60. Schembl12920083
61. Canthaxanthin (e 161g)
62. Ci 40850 [inci]
63. Hms2089k15
64. Hy-b1960
65. Tox21_111533
66. All-trans-beta-carotene-4,4'-dione
67. Canthaxanthin, >=95.0% (hplc)
68. E-161g
69. Lmpr01070264
70. Nsc374110
71. Zinc17653971
72. .beta.-carotene-4,4'-dione
73. 4,4'-diketo-.beta.-carotene
74. Ccg-207976
75. Cs-6879
76. Cas-514-78-3
77. Ls-15425
78. Ro-19915
79. Canthaxanthin (trans), Analytical Standard
80. Canthaxanthine 10 Microg/ml In Acetonitrile
81. Ci-(1975)no.40850
82. C08583
83. Ab00053349-02
84. Q385657
85. W-105885
86. Canthaxanthine. Short Expiry Date Due To Chemical Nature Of Component(s)
87. 3,3'-((1e,3e,5e,7e,9e,11e,13e,15e,17e)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,4,4-trimethylcyclohex-2-enone)
| Molecular Weight | 564.8 g/mol |
|---|---|
| Molecular Formula | C40H52O2 |
| XLogP3 | 11.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 10 |
| Exact Mass | 564.396730897 g/mol |
| Monoisotopic Mass | 564.396730897 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 9 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)