

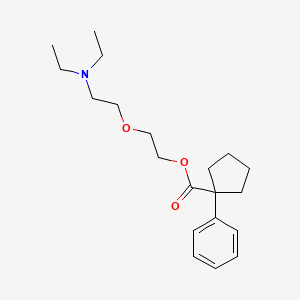

1. 2-(2-diethylaminoethoxy)ethyl 1-phenylcyclopentyl-1-carboxylate
2. Carbetapentane 1,5-napthalenedisulfonate (2:1)
3. Carbetapentane 2,6-napthalenedisulfonate (2:1)
4. Carbetapentane Citrate
5. Carbetapentane Tannate
6. Pentoxyverine
1. Pentoxyverine
2. 77-23-6
3. Pentoxyverin
4. Pentoxiverin
5. Atussil
6. U.c.b. 2543
7. Chembl73234
8. 2-(2-diethylaminoethoxy)ethyl 1-phenylcyclopentanecarboxylate
9. 2-(diethylaminoethoxy)ethyl 1-phenyl-1-cyclopentanecarboxylate
10. 2-(diethylaminoethoxy)ethyl 1-phenylcyclopentyl-1-carboxylate
11. 2-(2-(diethylamino)ethoxy)ethyl 1-phenylcyclopentanecarboxylate
12. 1-phenylcyclopentane-1-carboxylic Acid Diethylaminoethoxyethyl Ester
13. 2-[2-(diethylamino)ethoxy]ethyl 1-phenylcyclopentane-1-carboxylate
14. 1-phenylcyclopentanecarboxylic Acid,2-(2-(diethylamino)ethoxy)ethyl Ester
15. Cyclopentanecarboxylic Acid, 1-phenyl-, 2-(2-(diethylamino)ethoxy)ethyl Ester
16. Ethanol, 2-(2-(diethylamino)ethoxy)-, 1-phenylcyclopentanecarboxylate (ester)
17. Pentoxyverine (inn)
18. 32c726x12w
19. Ncgc00024595-03
20. Pentoxiverinum
21. Sedotussin (tn)
22. 2-[2-(diethylamino)ethoxy]ethyl 1-phenylcyclopentanecarboxylate
23. Dsstox_cid_2734
24. Cyclopentanecarboxylic Acid, 1-phenyl-, 2-[2-(diethylamino)ethoxy]ethyl Ester
25. Pentoxyverine [inn]
26. Dsstox_rid_76706
27. Dsstox_gsid_22734
28. Pentoxiverina
29. Pentoxiverine
30. Pentoxyverinum
31. Pentoxyverine [inn:ban]
32. Cas-77-23-6
33. Pentoxyverinum [inn-latin]
34. Pentoxiverina [inn-spanish]
35. Hsdb 3299
36. 1-phenyl-1-cyclopentanecarboxylate
37. Ucb 2543
38. Einecs 201-014-1
39. Brn 2299701
40. Pentoxyverine (base)
41. Sedotussin
42. Unii-32c726x12w
43. Ethanol, 2-[2-(diethylamino)ethoxy]-, 1-phenylcyclopentanecarboxylate (ester)
44. Carbapentane
45. Toclase (salt/mix)
46. Tuclase (salt/mix)
47. Spectrum_001366
48. Spectrum_001952
49. Tocris-0454
50. Sedotussin (salt/mix)
51. Prestwick0_000387
52. Prestwick1_000387
53. Prestwick2_000387
54. Prestwick3_000387
55. Spectrum2_001412
56. Spectrum3_000922
57. Spectrum4_001021
58. 1-phenylcyclopentanecarboxylic Acid 2-(2-diethylaminoethoxy)ethyl Ester
59. Carbetapentane [mi]
60. Cyclopentanecarboxylic Acid, 1-phenyl-, 2-(2-(diethylamino)ethoxy)ethylester
61. Pentoxyverine [hsdb]
62. Lopac0_000313
63. Schembl67879
64. Bspbio_000573
65. Kbiogr_001541
66. Kbioss_001846
67. Kbioss_002506
68. Cid_90010
69. Carbetapentane [vandf]
70. Divk1c_000356
71. Pentoxyverine [mart.]
72. Spbio_001484
73. Spbio_002494
74. Pentoxyverine [who-dd]
75. Bpbio1_000631
76. Dtxsid9022734
77. Bdbm94507
78. Chebi:94484
79. Kbio1_000356
80. Kbio2_001846
81. Kbio2_002498
82. Kbio2_004414
83. Kbio2_005066
84. Kbio2_006982
85. Kbio2_007634
86. Kbio3_001924
87. Ninds_000356
88. Zinc3830375
89. Tox21_110909
90. 2,2-dimethyl-7-octenoicacid
91. Pdsp1_001673
92. Pdsp2_001656
93. 1-phenylcyclopentanecarboxylic Acid 2-[2-(diethylamino)ethoxy]ethyl Ester
94. Akos015918339
95. Tox21_110909_1
96. Ccg-204408
97. Db11186
98. Sdccgsbi-0050301.p004
99. Idi1_000356
100. Ncgc00024595-01
101. Ncgc00024595-02
102. Ncgc00024595-04
103. Ncgc00024595-05
104. Ncgc00024595-06
105. Ncgc00024595-08
106. Ncgc00024595-15
107. Sbi-0050301.p003
108. Db-056191
109. Hy-134004
110. Ab00053602
111. Cs-0136428
112. Ft-0603066
113. S0869
114. D08334
115. Ab00053602_09
116. Q174786
117. W-104325
118. Brd-k06181161-048-04-5
119. Brd-k06181161-048-07-8
120. 1-cyclopentanecarboxylate, 2-(diethylaminoethoxy)ethyl-1-phenyl-
121. 2-[2-(diemthylamino)ethoxy]ethyl 1-phenylcyclopentanecarboxylate
122. 2-{[2-(diethylamino)ethyl]oxy}ethyl 1-phenylcyclopentanecarboxylate
123. 1-phenyl-1-cyclopentanecarboxylic Acid 2-[2-(diethylamino)ethoxy]ethyl Ester
124. Citric Acid;1-phenylcyclopentanecarboxylic Acid 2-[2-(diethylamino)ethoxy]ethyl Ester
125. 2-(2-diethylaminoethoxy)ethyl 1-phenylcyclopentane-1-carboxylate; 2-hydroxypropane-1,2,3-tricarboxylic Acid
126. 2-[2-(diethylamino)ethoxy]ethyl 1-phenylcyclopentane-1-carboxylate;2-oxidanylpropane-1,2,3-tricarboxylic Acid
127. 2-hydroxypropane-1,2,3-tricarboxylic Acid;1-phenyl-1-cyclopentanecarboxylic Acid 2-[2-(diethylamino)ethoxy]ethyl Ester
| Molecular Weight | 333.5 g/mol |
|---|---|
| Molecular Formula | C20H31NO3 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 11 |
| Exact Mass | 333.23039385 g/mol |
| Monoisotopic Mass | 333.23039385 g/mol |
| Topological Polar Surface Area | 38.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 356 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antitussive Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...DRUGS THAT HAVE BEEN USED AS CENTRALLY ACTING ANTITUSSIVES INCL...CARBETAPENTANE...
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 530
OTHER DRUGS THAT HAVE BEEN USED AS...ANTITUSSIVES INCL...CARBETAPENTANE, CARAMIPHEN, & OXOLAMINE. ...IN GENERAL THEIR TOXICITY IS LOW, BUT CONTROLLED CLINICAL STUDIES ARE STILL INSUFFICIENT TO DETERMINE WHETHER THEY MERIT CONSIDERATION AS ALTERNATIVES TO MORE THOROUGHLY STUDIED AGENTS.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 530
GENERALLY, ANY CENTRALLY ACTING ANTITUSSIVE SHOULD BE GIVEN CAUTIOUSLY WITH OTHER CENTRALLY ACTING AGENTS. /ANTITUSSIVES/
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 976
Indicated as a cough suppressant to relieve cough caused by the common cold, flu, bronchitis, and sinusitis.
Pentoxyverine induces an antitussive action. In animal studies, intraperitoneal administration of pentoxyverine inhibited citric-acid-induced cough in guinea-pigs _in vivo_. Some mice and rat studies suggest that pentoxyverine may also exert anticonvulsant activities without inducing a protective effect from NMDA-induced lethality. Protective effects against maximal electroshock-induced seizures in a dose-related fashion was also observed following either intraperitoneal or oral administration. In hERG-transfected cells, pentoxyverine inhibited the outward current of the hERG ion channel with half-maximal inhibition concentrations (IC50) of 3.0 M. In rats receiving intrathecal administration, pentoxyverine exhibited dose-dependent spinal blockade with a more sensory-selective action over motor blockade. It induced a spinal blockade with a more sensory/nociceptive-selective action over motor blockade compared to lidocaine.
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
R - Respiratory system
R05 - Cough and cold preparations
R05D - Cough suppressants, excl. combinations with expectorants
R05DB - Other cough suppressants
R05DB05 - Pentoxyverine
Absorption
In humans, maximum plasma concentrations are achieved 1.2 hours after oral dosing.
Route of Elimination
No pharmacokinetic data available.
Volume of Distribution
No pharmacokinetic data available.
Clearance
No pharmacokinetic data available.
No pharmacokinetic data available.
The half-life is 2.3 hours following oral dosing.
While the mechanism of antitussive action of pentoxyverine is not fully understood, it is thought to be mediated via sigma-1 receptors expressed in the central nervous system. Pentoxyverine acts as an agonist at sigma receptors with the Ki of 7528 nM, as demonstrated in a competitive binding assay. The function of sigma receptors on cough suppressant activities is unclear, however these receptors are highly expressed in the nucleus tractus solitarius (NTS) of the brainstem where the afferent fibres first synapse. NTS is located very close to the cough centre in the brainstem thus may function as a gate' for the cough reflex and allow sigma-1 receptor agonists to modulate afferent activity prior to reaching the cough center. It is suggested that highly lipophilic sigma-1 agonists may penetrate the CNS following systemic administration. When administered as aerosols, sigma-1 receptor agonists may temporarily act in the periphery to modulate cough by acting activate sigma receptors expressed in the lungs. However there is limited evidence of peripheral localization of the sigma agonists following aerosol administration and the ruling out of systemic exposure. The local anesthesia action of pentoxyverine may occur through inhibition of voltage-gated Na(+) currents.
NUMBER OF DRUGS ARE KNOWN TO REDUCE COUGH AS RESULT OF THEIR CENTRAL ACTIONS, ALTHOUGH EXACT MECHANISMS ARE STILL NOT ENTIRELY CLEAR. /NONOPIOID ANTITUSSIVES/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 530