
1. Monoxide, Carbon
1. Carbon Monooxide
2. 630-08-0
3. Carbonic Oxide
4. Carbon Oxide (co)
5. Carbon(ii) Oxide
6. Carbon Monoxide [usan]
7. Carboneum Oxygenisatum
8. [co]
9. Chebi:17245
10. 7u1ee4v452
11. Carbon Monoxide (usan)
12. Kohlenoxyd [german]
13. Koolmonoxyde [dutch]
14. Monoxide
15. Kohlenmonoxid [german]
16. Wegla Tlenek [polish]
17. Oxyde De Carbone [french]
18. C#o
19. Carbone (oxyde De) [french]
20. Hsdb 903
21. Carbonio (ossido Di) [italian]
22. Einecs 211-128-3
23. Na9202
24. Un1016
25. Unii-7u1ee4v452
26. .carbon Monoxide
27. Carbon Monoxide 10% By Volume Or More
28. Carbon Monoxide, Compressed
29. Ec 211-128-3
30. Carbon Monoxide [mi]
31. Carbon Monoxide [hsdb]
32. Carbon Monoxide [vandf]
33. Carbon Monoxide [mart.]
34. Chembl1231840
35. Dtxsid5027273
36. Carbon Monoxide [who-dd]
37. C0369
38. Carboneum Oxygenisatum [hpus]
39. Carbon Monoxide [ep Monograph]
40. Db11588
41. Q2025
42. C00237
43. D09706
44. Carbon Monoxide, Compressed [un1016] [poison Gas]
45. Carbon Monoxide, Refrigerated Liquid (cryogenic Liquid)
46. Carbon Monoxide, Refrigerated Liquid (cryogenic Liquid) [na9202] [poison Gas]
| Molecular Weight | 28.010 g/mol |
|---|---|
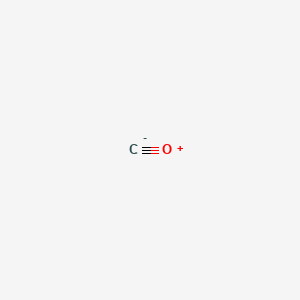
| Molecular Formula | CO |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 27.994914619 g/mol |
| Monoisotopic Mass | 27.994914619 g/mol |
| Topological Polar Surface Area | 1 Ų |
| Heavy Atom Count | 2 |
| Formal Charge | 0 |
| Complexity | 10 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): Euthanasia of dogs and cats can be carried out in a carbon monoxide chamber, but there are a number of precautions and guidelines for proper use of such chambers.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 1061
There are over 350 variants to normal human hemoglobin. In the hemoglobin S variant, sickling takes place when deoxyhemoglobin S in the red blood cell reaches a critical level and causes intracellular polymerization. Oxygenation of the hemoglobin S molecules in the polymer, therefore, should lead to a change in molecular shape, breakup of the polymer and unsickling of the cell. Carbon monoxide was considered at one time to be potentially beneficial, because it ultimately would reduce the concentration of deoxyhemoglobin S by converting part of the hemoglobin to carboxyhemoglobin. Exposure to carbon monoxide, however, was not considered to be an effective clinical treatment, because high carboxyhemoglobin levels (>20%) were required. /Former use/
Environmental Health Criteria 213: Carbon Monoxide pp. 1-12 (1999) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization
/EXPER THER/ Cardiopulmonary bypass (CPB) is associated with pulmonary inflammation and dysfunction. This may lead to acute lung injury and acute respiratory distress syndrome with increased morbidity and mortality. The authors hypothesized that inhaled carbon monoxide before initiation of CPB would reduce inflammatory response in the lungs. In a porcine model, a beating-heart CPB was used. The animals were either randomized to a control group, to standard CPB, or to CPB plus carbon monoxide. In the latter group, lungs were ventilated with 250 ppm inhaled carbon monoxide in addition to standard ventilation before CPB. Lung tissue samples were obtained at various time points, and pulmonary cytokine levels were determined. Hemodynamic parameters were largely unaffected by CPB or carbon monoxide inhalation. There were no significant differences in cytokine expression in mononuclear cells between the groups throughout the experimental time course. Compared with standard CPB animals, carbon monoxide significantly suppresses tumor necrosis factor-alpha and interleukin-1beta levels (P<0.05) and induced the antiinflammatory cytokine interleukin 10 (P<0.001). Carbon monoxide inhalation modulates effector caspase activity in lung tissue during CPB. The results demonstrate that inhaled carbon monoxide significantly reduces CPB-induced inflammation via suppression of tumor necrosis factor alpha, and interleukin-1beta expression and elevation of interleukin 10. Apoptosis induced by CPB was associated with caspase-3 activation and was significantly attenuated by carbon monoxide treatment. Based on the observations of this study, inhaled carbon monoxide could represent a potential new therapeutic modality for counteracting CPB-induced lung injury.
PMID:18497603 Goebel U et al; Anesthesiology 108 (6): 1025-36 (2008)
Used as a marker of respiratory status in spirometry tests,. Food additive for pigment fixation in meat.
Carbon monoxide is used to measure the diffusing capacity for carbon monoxide (DLCO), also known as the transfer factor for carbon monoxide. It is a measure of the gas transfer from inspired gas to the circulatory system (red blood cells in particular). It is used in a particular pulmonary function test called "the single-breath test". DLCO, measured for clinical and research purposes almost exclusively by the single-breath method is an important and very useful pulmonary function test. It is useful in the evaluation of patients with dyspnea, obstructive lung diseases, restrictive lung diseases, and in patients with diseases of the pulmonary vasculature. The measurement of DLCO using carbon monoxide is representative of the surface area available, the volume of blood present in the pulmonary capillaries, as well as the thickness of the alveolar-capillary membrane. **Conditions that increase DLCO:** Heart failure, erythrocythemia, alveolar hemorrhage, asthma **Conditions that decrease DLCO:** emphysema, pulmonary fibrosis, pulmonary hypertension, pulmonary embolism In addition to the above uses, carbon monoxide (CO) is increasingly being accepted in recent years as a protective molecule with important signaling capabilities in both physiological/homeostatic and pathophysiological situations. The endogenous production of CO occurs via the activity of constitutive (heme oxygenase 2) and inducible (heme oxygenase 1) heme oxygenase enzymes, which are both responsible for the breakdown of heme. Through the generation of its products, which in addition to carbon monoxide, includes the biliary pigments biliverdin, bilirubin and ferrous iron, the heme oxygenase 1 system also have an essential role in the regulation of the stress response and in cell adaptation to injury. Preclinical studies have suggested potential benefits of carbon monoxide in cardiovascular disease, inflammatory disorders, as well as organ transplantation.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Gasotransmitters
Endogenously produced lipid-soluble gaseous molecules which function as neurotransmitters and signal mediators targeting ION CHANNELS and transporters. (See all compounds classified as Gasotransmitters.)
V - Various
V04 - Diagnostic agents
V04C - Other diagnostic agents
V04CX - Other diagnostic agents
V04CX08 - Carbon monoxide
Absorption
Although CO is not one of the respiratory gases, the similarity of physico-chemical properties of CO and oxygen (O2) permits an extension of the findings of studies on the kinetics of transport of O2 to those of CO. The rate of formation and elimination of COHb, its concentration in blood, and its catabolism is controlled by numerous physical factors and physiological mechanisms. The absorption of carbon monoxide from the consumption treated food products is not significant. Risk of CO toxicity from the packaging process or from consumption of CO-treated meats is negligible.
Carbon monoxide is eliminated through the lungs when air free of carbon monoxide is inhaled.
Sax, N.I. Dangerous Properties of Industrial Materials. 6th ed. New York, NY: Van Nostrand Reinhold, 1984., p. 643
... /Carbon monoxide/ readily crosses placenta.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1620
Carbon monoxide is not a cumulative poison in the usual sense. Carboxyhemoglobin is fully dissociable, and once exposure has been terminated, the pigment will revert to oxyhemoglobin. Liberated carbon monoxide is eliminated via the lungs.
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 268
The absorption of carbon monoxide is said not to occur, but its absorption followed by oxidation within the epidermis has not been excluded.
Hayes, W.J., Jr., E.R. Laws Jr., (eds.). Handbook of Pesticide Toxicology Volume 1. General Principles. New York, NY: Academic Press, Inc., 1991., p. 139
For more Absorption, Distribution and Excretion (Complete) data for Carbon monoxide (13 total), please visit the HSDB record page.
Metabolism of the dihalomethanes leads to dehalogenation, and the end product is carbon monoxide ... The carbon monoxide appears to arise from a formyl halide intermediate resulting from the loss of one halide atom from the halocarbon. This intermediate as an alternative to losing carbon monoxide can covalently bind to cellular protein or lipid.
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 692
In addition to exogenous sources, humans are also exposed to small amounts of carbon monoxide produced endogenously. In the process of natural degradation of hemoglobin to bile pigments, in concert with the microsomal reduced nicotinamide adenine dinucleotide phosphate (NADPH) cytochrome P-450 reductase, two heme oxygenase isoenzymes, HO-1 and HO-2, catalyse the oxidative breakdown of the alpha-methene bridge of the tetrapyrrole ring of heme, leading to the formation of biliverdin and carbon monoxide. The major site of heme breakdown, and therefore the major organ for production of endogenous carbon monoxide, is the liver. The spleen and the erythropoietic system are other important catabolic generators of carbon monoxide ... Other hemoproteins, such as myoglobin, cytochromes, peroxidases and catalase, contribute approximately 20-25% to the total amount of carbon monoxide generated. Approximately 0.4 mL carbon monoxide/hr is formed by hemoglobin catabolism, and about 0.1 mL/hr originates from non-hemoglobin sources.
Environmental Health Criteria 213: Carbon Monoxide pp. 1-12 (1999) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization.
Any disturbance leading to increased destruction of red blood cells and accelerated breakdown of other haemoproteins would lead to increased production of carbon monoxide. Hematomas, intravascular hemolysis of red blood cells, blood transfusion and ineffective erythropoiesis will all elevate the carbon monoxide concentration in the blood. Degradation of red blood cells under pathological conditions such as anemias (hemolytic, sideroblastic, sickle cell), thalassaemia, Gilbert's syndrome with hemolysis and other hematological diseases will also accelerate carbon monoxide production.
Environmental Health Criteria 213: Carbon Monoxide pp. 1-12 (1999) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization.
The primary factors that determine the final level of carboxyhemoglobin are: the amount of inspired carbon monoxide; minute alveolar ventilation at rest and during exercise; endogenous carbon monoxide production; blood volume; barometric pressure; and the relative diffusion capability of the lungs. The rate of diffusion from the alveoli and the binding of carbon monoxide with the blood hemoglobin are the steps limiting the rate of uptake into the blood.
WHO; Environ Health Criteria 13: Carbon Monoxide p.35 (1979)
For more Metabolism/Metabolites (Complete) data for Carbon monoxide (6 total), please visit the HSDB record page.
The half-life of carbon monoxide at room air temperature is 3-4 hours. 100% oxygen reduces the half-life to 30-90 minutes; hyperbaric oxygen at 2.5 atm (atmosphere units) with 100% oxygen reduces it to 15-23 minutes.
Elimination 1/2 life: 5-6 hours (shortened by administration of oxygen); [TDR, p. 283]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 283
The half-time of carbon monoxide disappearance from blood under normal recovery conditions while breathing air showed considerable between-individual variance. For carboxyhemoglobin concentrations of 2-10%, the half-time ranged from 3 to 5 hr; others reported the range to be 2-6.5 hr for slightly higher initial concentrations of carboxyhemoglobin.
Environmental Health Criteria 213: Carbon Monoxide pp. 1-12 (1999) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization.
The biological half-life of carbon monoxide concentration in the blood of sedentary adults is about 2-5 hr. The elimination of carbon monoxide becomes slower with time, and the lower the initial level of carboxyhemoglobin, the slower the rate of excretion.
International Labour Office. Encyclopedia of Occupational Health and Safety. Vols. I&II. Geneva, Switzerland: International Labour Office, 1983., p. 396
In respiratory testing, the diffusing capacity for carbon monoxide (DLCO) is a measure of the ability of gas to transfer from the alveoli across the alveolar epithelium and the capillary endothelium to the red blood cells. The DLCO depends not only on the area and thickness of the blood-gas barrier but additionally on the volume of blood in the pulmonary capillaries. The distribution of alveolar volume and ventilation also has an impact on the measurement. DLCO is measured by sampling end-expiratory gas for carbon monoxide (CO) after patients inspire a small and safe amount of exogenous CO, hold their breath, and exhale. Measured DLCO is adjusted for alveolar volume (which is estimated from dilution of helium) and the patients hematocrit level. DLCO is reported as mL/min/mm Hg and as a percentage of a predicted value. Carbon monoxide exerts effects on cell metabolism through both hypoxic and non-hypoxic modes of action. Both mechanisms of action are thought to be the result of the ability of carbon monoxide to bind strongly to heme and alter the function and/or metabolism of heme proteins. The binding affinity of carbon monoxide for hemoglobin is more than 200 times greater than that of oxygen for hemoglobin. The formation of carboxyhemoglobin (COHb) decreases the O2 carrying capacity of blood and disrupts the release of O2 from Hb for its use in tissues. Through similar mechanisms, carbon monoxide diminishes the O2 storage in muscle cells by binding to and displacing O2 from, myoglobin. Though all human tissues are vulnerable to carbon monoxide-induced hypoxic injury, those with the highest O2 demand are especially vulnerable, including the brain and heart. Most of the non-hypoxic mechanisms of action of carbon monoxide have been thought to be due to binding of carbon monoxide to heme in proteins other than Hb. The most notable targets of carbon monoxide include components of many important physiological regulatory systems, including brain and muscle oxygen storage and use(myoglobin, neuroglobin); nitric oxide cell signaling (e.g., nitric oxide synthase, guanylyl cyclase); prostaglandin cell signaling (cyclooxygenase, prostaglandin H synthase); energy metabolism and mitochondrial respiration (cytochrome c oxidase, cytochrome c, NADPH oxidase); steroid and drug metabolism (cytochrome P450); cellular redox balance and reactive oxygen species (ROS; catalase, peroxidases); and numerous transcription factors (e.g., neuronal PAS domain protein, NPAS2, implicated in regulation of circadian rhythm). In meat processing, carbon monoxide reacts with myoglobin, to form carboxymyoglobin, imparting a red appearance to the meat.
The binding of carbon monoxide to hemoglobin, producing carboxyhemoglobin and decreasing the oxygen carrying capacity of blood, appears to be the principal mechanism of action underlying the induction of toxic effects of low-level carbon monoxide exposures. The precise mechanisms by which toxic effects are induced via carboxyhemoglobin formation are not understood fully but likely include the induction of a hypoxic state in many tissues of diverse organ systems. Alternative or secondary mechanisms of carbon monoxide-induced toxicity (besides carboxyhaemoglobin) have been hypothesized, but none has been demonstrated to operate at relatively low (near-ambient) carbon monoxide exposure levels.
Environmental Health Criteria 213: Carbon Monoxide pp. 1-12 (1999) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization.
Carbon monoxide is known to react with a variety of metal-containing proteins found in nature. Carbon monoxide-binding metalloproteins present in mammalian tissues include oxygen carrier proteins such as hemoglobin and myoglobin as well as metalloenzymes (oxidoreductases) such as cytochrome c oxidase, cytochromes of the P-450 type, tryptophan oxygenase and dopamine hydroxylase. These metalloproteins contain iron and/or copper centers at their active sites that form metal-ligand complexes with carbon monoxide in competition with molecular oxygen. Carbon monoxide and oxygen form complexes with metalloenzymes only when the iron and copper are in their reduced forms (Fe(II), Cu(I)). ... The competitive relationship between carbon monoxide and oxygen for the active site of intracellular hemoproteins is usually described by the Warburg partition coefficient (R), which is the carbon monoxide/oxygen ratio that produces 50% inhibition of the oxygen uptake of the enzyme, or, in the case of myoglobin, a 50% decrease in the number of available oxygen binding sites.
Environmental Health Criteria 213: Carbon Monoxide pp. 1-12 (1999) by the International Programme on Chemical Safety (IPCS) under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation and the World Health Organization
Carbon monoxide ... reacts /in the blood stream/ with hemoglobin to form carboxyhemoglobin, a form which is incapable of combining with oxygen. Exposure to air containing 0.4% of carbon monoxide for 20-30 min results in the conversion of 70% of the hemoglobin in the blood to carboxyhemoglobin.
Humphreys, D.J. Veterinary Toxicology. 3rd ed. London, England: Bailliere Tindell, 1988., p. 81
Carbon monoxide binds tightly to the reduced form of iron in hemoglobin, reducing the delivery of oxygen to tissues. Although this has for many years been thought to be the sole mechanism of toxicity of carbon monoxide, there is evidence to suggest that carbon monoxide also binds to cytochrome a + a3.
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 28
For more Mechanism of Action (Complete) data for Carbon monoxide (8 total), please visit the HSDB record page.