
1. Carminomicin
2. Carminomycin I
3. Carminomycin Ii
4. Carminomycin Iii
5. Carubicin
6. Carubicin Hydrochloride
7. Demethyldaunomycin
8. Demethyldaunorubicin
9. Hydrochloride, Carubicin
10. Karminomicin
11. Karminomycin
12. Nsc 180,024
13. Nsc 180024
14. Nsc-180,024
15. Nsc-180024
16. Nsc180,024
17. Nsc180024
18. Rubeomycin A
19. Rubeomycin A1
1. Carubicin
2. 39472-31-6
3. Carminomycin I
4. Karminomycin
5. 50935-04-1
6. Carubicin [inn]
7. O-demethyldaunomycin
8. Carminomicin I
9. Nsc-180024
10. Ccris 961
11. Chebi:31359
12. Dsstox_cid_2742
13. Dsstox_rid_76711
14. Dsstox_gsid_22742
15. Karminomitsin
16. Ncgc00159344-02
17. 50935-04-1 (free Base)
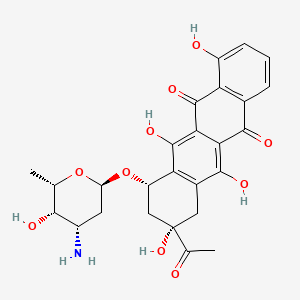
18. (7s,9s)-9-acetyl-7-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7h-tetracene-5,12-dione
19. E7437k3983
20. (8s,10s)-8-acetyl-10-(((2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-1,6,8,11-tetrahydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione
21. Carubicina
22. Carubicine
23. Carubicinum
24. (1s,3s)-3-acetyl-3,5,10,12-tetrahydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranoside
25. (7s,9s)-9-acetyl-7-[(2r,4s,5s,6s)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-4,6,9,11-tetrahydroxy-8,10-dihydro-7h-tetracene-5,12-dione
26. 5,12-naphthacenedione,8-acetyl-10-[(3-amino-2,3,6-trideoxy-a-l-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8s,10s)-
27. Cas-50935-04-1
28. Carubicine [inn-french]
29. Carubicinum [inn-latin]
30. Carubicina [inn-spanish]
31. Ccris 6185
32. Unii-e7437k3983
33. Ncgc00160675-01
34. Carminomycin; Carubicin
35. Carubicin [mi]
36. Schembl9552
37. Chembl474260
38. Dtxsid3022742
39. Ex-a2244
40. Hy-b2171
41. Zinc4654755
42. Tox21_111589
43. Tox21_111978
44. Bdbm50103635
45. (1s,3s)-3-acetyl-1,2,3,4,6,11-hexahydro-3,5,10,12-tetrahydroxy-6,11-dioxo-1-naphthacenyl-3-amino-2,3,6-tridesoxy-alpha-l-lyxo-hexopyranosid
46. Cs-0021097
47. Carminomycin, O-demethyldaunomycin, Ccris 961
48. 935c041
49. A857268
50. Q5047474
51. (1s,3s)-3-acetyl-1,2,3,4,6,11-hexahydro-3,5,10,12-tetrahydroxy-6,11-dioxo-1-naphthacenyl 3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranoside
52. 5,12-naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8s-cis)-
53. 5,12-naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8s-cis)-
54. 5,12-naphthacenedione, 8-acetyl-10-[(3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-1,6,8,11-tetrahydroxy-, (8s,10s)-
| Molecular Weight | 513.5 g/mol |
|---|---|
| Molecular Formula | C26H27NO10 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 3 |
| Exact Mass | 513.16349606 g/mol |
| Monoisotopic Mass | 513.16349606 g/mol |
| Topological Polar Surface Area | 197 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 944 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Topoisomerase II Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE II. Included in this category are a variety of ANTINEOPLASTIC AGENTS which target the eukaryotic form of topoisomerase II and ANTIBACTERIAL AGENTS which target the prokaryotic form of topoisomerase II. (See all compounds classified as Topoisomerase II Inhibitors.)
Antibiotics, Antineoplastic
Chemical substances, produced by microorganisms, inhibiting or preventing the proliferation of neoplasms. (See all compounds classified as Antibiotics, Antineoplastic.)