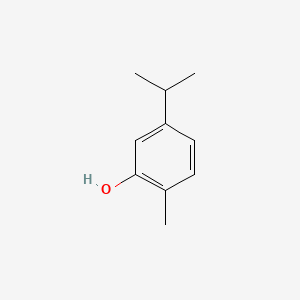

1. 5-isopropyl-2-methylphenol
1. 5-isopropyl-2-methylphenol
2. 499-75-2
3. Isopropyl-o-cresol
4. O-thymol
5. Antioxine
6. Karvakrol
7. 2-p-cymenol
8. 5-isopropyl-o-cresol
9. Isothymol
10. 2-hydroxy-p-cymene
11. Phenol, 2-methyl-5-(1-methylethyl)-
12. P-cymen-2-ol
13. 2-methyl-5-isopropylphenol
14. 5-isopropyl-2-methyl-phenol
15. 2-methyl-5-(1-methylethyl)phenol
16. 2-methyl-5-(propan-2-yl)phenol
17. 2-methyl-5-propan-2-ylphenol
18. 3-isopropyl-6-methylphenol
19. P-cymene, 2-hydroxy-
20. O-cresol, 5-isopropyl-
21. 1-hydroxy-2-methyl-5-isopropylbenzene
22. 6-methyl-3-isopropylphenol
23. Phenol, 5-isopropyl-2-methyl-
24. Cymophenol
25. Fema No. 2245
26. Oxycymol
27. 1-methyl-2-hydroxy-4-isopropylbenzene
28. Phenol, 3-isopropyl-6-methyl-
29. 2-hydroxycymene
30. Nsc 6188
31. Chebi:3440
32. Chembl281202
33. 9b1j4v995q
34. Nsc-6188
35. 2-methyl-5-(1-methylethyl)-phenol
36. Cymene-2-ol, P-
37. Caswell No. 511
38. Cymenol
39. Ccris 7450
40. Hsdb 906
41. Einecs 207-889-6
42. Epa Pesticide Chemical Code 022104
43. Brn 1860514
44. Unii-9b1j4v995q
45. Ai3-03438
46. Hydroxy-p-cymene
47. Carvacrol Natural
48. Carvacrol,(s)
49. P-cymene-2-ol
50. Carvacrol, 98%
51. Mfcd00002236
52. Dentol
53. Carvacrol [fcc]
54. Carvacrol [mi]
55. Carvacrol [fhfi]
56. Carvacrol [hsdb]
57. Carvacrol [inci]
58. Carvacrol [who-dd]
59. Dsstox_cid_22074
60. Dsstox_rid_79916
61. Dsstox_gsid_42074
62. Schembl24734
63. 3-isopropyl-6-methyl Phenol
64. 3-isopropyl-6-methyl-phenol
65. 4-06-00-03331 (beilstein Handbook Reference)
66. Bidd:er0492
67. Carvacrol, Analytical Standard
68. Gtpl2497
69. Carvacrol, Natural, 99%, Fg
70. Dtxsid6042074
71. Methyl-5-(1-methylethyl)phenol
72. P-mentha-1,3,5-trien-2-ol
73. Wln: Qr B1 Ey1&1
74. Fema 2245
75. Nsc6188
76. Carvacrol, >=98%, Fcc, Fg
77. Zinc967563
78. Hy-n0711
79. Tox21_301378
80. Bdbm50240433
81. S3788
82. Stl453136
83. Akos000120828
84. Ac-2688
85. Ccg-266210
86. Fs-4199
87. Lmpr0102090017
88. Mb00118
89. Ncgc00256001-01
90. Cas-499-75-2
91. Isothymol (=2-isopropyl-4-methyl Phenol)
92. Carvacrol Cymenol 5-isopropyl-2-methylphenol
93. Cs-0009729
94. Ft-0627526
95. 2-hydroxy-4-isopropyl-1-methylbenzene
96. C09840
97. F17722
98. A827907
99. Q225543
100. Carvacrol, Primary Pharmaceutical Reference Standard
101. W-105999
102. Benzene,2-hydroxy,4-isopropyl,1-methyl Carvacrol
103. F8889-1978
104. Z1317839236
105. Benzene,2-hydroxy,4-isopropyl,1-methyl Carvacrol
| Molecular Weight | 150.22 g/mol |
|---|---|
| Molecular Formula | C10H14O |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 150.104465066 g/mol |
| Monoisotopic Mass | 150.104465066 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
4. 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG, BETWEEN 1 TEASPOON & 1 OZ FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-126
CARVACROL APPEARS TO BE SLOWLY ABSORBED FROM INTESTINE IN RABBIT, SINCE 22 HR AFTER ADMIN OF 1.5 G, SOME 30% WAS STILL IN GASTRO-INTESTINAL TRACT, ABOUT 25% OF DOSE HAVING BEEN EXCRETED IN URINE.
OPDYKE DL J; MONOGRAPHS ON FRAGRANCE RAW MATERIALS. CARVACROL; FOOD COSMET TOXICOL 17 (SUPPL) 743 (1979)
CARVACROL APPLIED TO INTACT SHAVED ABDOMINAL SKIN OF MOUSE WAS NOT ABSORBED WITHIN 2 HR.
OPDYKE DL J; MONOGRAPHS ON FRAGRANCE RAW MATERIALS. CARVACROL; FOOD COSMET TOXICOL 17 (SUPPL) 743 (1979)
/WHEN FED TO RABBITS,/ THE SUBSTITUTED MONOPHENOLS, THYMOL & CARVACROL, WHICH OCCUR IN ESSENTIAL OILS OF PLANTS...ARE...CONJUGATED WITH GLUCURONIC ACID & SULFATE.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 147
CARVACROL IS METABOLIZED BY CONJUGATION WITH GLUCURONIC ACID & SULFATE.
OPDYKE DL J; MONOGRAPHS ON FRAGRANCE RAW MATERIALS. CARVACROL; FOOD COSMET TOXICOL 17 (SUPPL) 743 (1979)
Carvacrol has known human metabolites that include (2-methyl-5-propan-2-ylphenyl) hydrogen sulfate and (2S,3S,4S,5R)-3,4,5-trihydroxy-6-(2-methyl-5-propan-2-ylphenoxy)oxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560