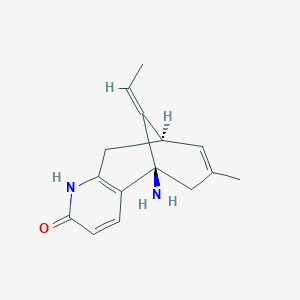

1. Huperzine A, (5alpha,9beta,11z)-(-)-isomer
1. (-)-huperzine A
2. 102518-79-6
3. (-)-huperazine A
4. Selagine
5. L-huperzine A
6. (-)-selagine
7. Hup A
8. Chembl395280
9. Chebi:78330
10. (5r,9r,e)-5-amino-11-ethylidene-7-methyl-5,6,9,10-tetrahydro-5,9-methanocycloocta[b]pyridin-2(1h)-one
11. Dsstox_cid_26038
12. Dsstox_rid_81302
13. Dsstox_gsid_46038
14. 0111871i23
15. Huperizine A
16. (+)-huperzine A
17. (5r,9r,11e)-5-amino-11-ethylidene-7-methyl-5,6,9,10-tetrahydro-5,9-methanocycloocta[b]pyridin-2(1h)-one
18. Cas-102518-79-6
19. (-)-huperzine A (hupa)
20. 120786-18-7
21. Cerebra
22. Hyperazzine A
23. Kimpukan A
24. 1vot
25. Ncgc00159362-02
26. (-)-huperazinea
27. Hup
28. Huperaine A
29. Huperzine A [mi]
30. Huperzine A [vandf]
31. Huperzine A [who-dd]
32. Schembl136368
33. Spectrum1505255
34. (−)-huperzine A
35. Dtxsid8046038
36. Chebi:94624
37. Zinc9411213
38. Tox21_111603
39. Bdbm50199522
40. Ccg-40292
41. Unii-0111871i23
42. Akos016842839
43. Tox21_111603_1
44. (+-)-ha
45. Db04864
46. Sdccgmls-0066755.p001
47. Ncgc00263655-01
48. As-15723
49. H1700
50. Q425198
51. Q-100029
52. Brd-k62240499-001-02-6
53. (-)-1-amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one(huperzine A)
54. (-)1-amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one( (-)-huperzine A)
55. (1r,9r)-1-amino-13-eth-(e)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one
56. (5r,9r,11e)-5-amino-11-ethylidene-5,6,9,10-tetrahydro-7-methyl-5,9-methanocycloocta(b)pyridin-2(1h)-one
57. (huperazine A)(-)-1-amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one
58. (r)-1-amino-13-eth-(e)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one
59. (r)-1-amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one
60. 1-amino-13-eth-(e)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one
61. 1-amino-13-eth-(z)-ylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one
62. 1-amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one
63. 1-amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one((+/-)-huperzine A)
64. 1-amino-13-ethylidene-11-methyl-6-aza-tricyclo[7.3.1.0*2,7*]trideca-2(7),3,10-trien-5-one((-)-huperzine A)
65. 1-amino-13-ethylidene-11-methyl-6-azatricyclo[7.3.1.0~2,7~]trideca-2(7),3,10-trien-5-one
66. 1369-64-8
67. 5,9-methanocycloocta(b)pyridin-2(1h)-one, 5-amino-11-ethylidene-5,6,9,10-tetrahydro-7-methyl-, (5r-(5-alpha,9-beta,11e))-
| Molecular Weight | 242.32 g/mol |
|---|---|
| Molecular Formula | C15H18N2O |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 242.141913202 g/mol |
| Monoisotopic Mass | 242.141913202 g/mol |
| Topological Polar Surface Area | 55.1 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 551 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in alzheimer's disease.
Huperzine A is an alkaloid derived from Huperzia serrata (which is available as an herbal product in the US). It is under investigation as an acetylcholinesterase inhibitor. Clinical trials in China have shown that huperzine A is comparably effective to the drugs currently on the market, and may even be somewhat safer in terms of side effects.
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
Huperzine A has been found to be an inhibitor of the enzyme acetylcholinesterase. This is the same mechanism of action of pharmaceutical drugs such as [galantamine] and [donepezil] used to treat Alzheimer's disease.