

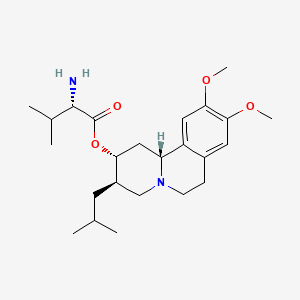

1. (2r,3r,11br)-9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2h- Benzo(a)quinolizin-2-yl L-valinate
2. Ingrezza
3. Nbi-98854
4. Valine 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2h-benzo(a)quinolizin-2-yl Ester
1. 1025504-45-3
2. Ingrezza
3. Mt-5199
4. Nbi 98854
5. 54k37p50kh
6. (2r,3r,11br)-9,10-dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2h- Benzo(a)quinolizin-2-yl L-valinate
7. L-valine, (2r,3r,11br)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2h-benzo(a)quinolizin-2-yl Ester
8. L-valine, (2r,3r,11br)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2h-benzo[a]quinolizin-2-yl Ester
9. Valbenazine [usan]
10. Valbenazine [usan:inn]
11. Unii-54k37p50kh
12. Valbenazine [mi]
13. Valbenazinenbi-98854
14. Valbenazine [inn]
15. Valbenazine (usan/inn)
16. Valbenazine [who-dd]
17. Gtpl8694
18. Chembl2364639
19. Schembl15932979
20. Dtxsid801026306
21. Ex-a2002
22. Bdbm50573733
23. Mfcd28963976
24. Zinc43195697
25. Cs-5908
26. Db11915
27. Ncgc00522306-02
28. [(2r,3r,11br)-9,10-dimethoxy-3-(2-methylpropyl)-2,3,4,6,7,11b-hexahydro-1h-benzo[a]quinolizin-2-yl] (2s)-2-amino-3-methylbutanoate
29. Ac-30929
30. As-35294
31. Hy-16771
32. Valine 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2h-benzo(a)quinolizin-2-yl Ester
33. D10675
34. Nbi-98854;nbi98854;nbi 98854
35. Q27089118
36. (2r,3r,11br)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-yl L-valinate
37. (s)-2-amino-3-methyl-butyric Acid (2r,3r,11br)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-yl Ester
38. [(2r,3r,11br)-9,10-dimethoxy-3-(2-methylpropyl)-2,3,4,6,7,11b-hexahydro-1h-pyrido[2,1-a]isoquinolin-2-yl] (2s)-2-amino-3-methylbutanoate
| Molecular Weight | 418.6 g/mol |
|---|---|
| Molecular Formula | C24H38N2O4 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 8 |
| Exact Mass | 418.28315770 g/mol |
| Monoisotopic Mass | 418.28315770 g/mol |
| Topological Polar Surface Area | 74 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 569 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of tardive dyskinesia in adults.
FDA Label
Valbenazine decreases the availability of monoamine neurotransmitters by preventing their storage in synaptic vesicles. This is believed to be the reason behind its therapeutic effect in tardive dyskinesia although the exact mechanism is unknown.
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX13 - Valbenazine
Absorption
Oral bioavailability of 49%. Tmax of 0.5-1h.
Route of Elimination
Roughly 60% is excreted in urine and 30% in feces. Less than 2% if the parent compound or active metabolite was excreted unchanged.
Volume of Distribution
92 Liters.
Clearance
7.2 Liters/hour.
Valbenzine is extensively metabolized to one active metabolite [+]--dihydrotetrabenazine ([+]--HTBZ) through hydrolysis of the valine ester reaching Cmax within 4-8 hours. It is also metabolized via oxidation by CYP3A4/5 to a mono-oxidzed metabolite NBI-136110 which also appears to pharmacologically active. [+]--HTBZ is metabolized by CYP2D6.
Both valbenazine and its active metabolite [+]--HTBZ have a half life of 15-22 hours.
Valbenazine and its active meabolites bind to and inhibit vesicular monoamine transporter 2 (VMAT2)with high selectivity (valbenazine Ki = 150nM, [+]--HTBZ Ki = 1.98nM, NBI136110 Ki = 160nM) with no significant binding to VMAT1 (Ki <10microM for each). This prevents the reuptake and storage of monoamine neurotransmitters noradrenaline, dopamine, and serotonin in synaptic vesicles making them vulnerable to metabolism by cytosolic enzymes. The presynaptic release of monoamine neurotransmitters is decreased due to the lack of vesicles with packaged neurotransmitter ready for release into the synapse. Neither valbenazine nor its active metabolite exhibit significant off target binding at dopamine, serotonin, or adrenaline receptors or uptake transporters at 10microM concentrations.