

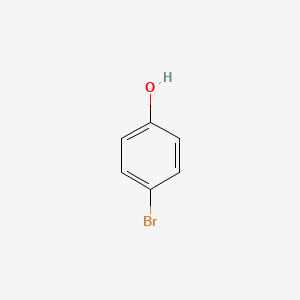

1. P-bromophenol
2. Para-bromophenol
1. 106-41-2
2. P-bromophenol
3. Phenol, 4-bromo-
4. P-bromohydroxybenzene
5. 4-bromo-phenol
6. Phenol, P-bromo-
7. 4-bromphenol
8. Para-bromophenol
9. 4-bromo Phenol
10. Mfcd00002313
11. Chembl57284
12. P-bromophenic Acid
13. Lao4j0183i
14. Nsc-4970
15. Bml
16. Ccris 632
17. Nsc 4970
18. Einecs 203-394-4
19. Parabromophenol
20. Parabromphenol
21. Unii-lao4j0183i
22. 4bromophenol
23. Para Bromophenol
24. Ai3-14903
25. P-bromo Phenol
26. P-bromo-phenol
27. 4-bromopbenol
28. Bromophenol-4
29. 4-bromanylphenol
30. Hsdb 7650
31. 1,4-bromophenol
32. 4-bromohydroxybenzene
33. 4-bromophenol, 99%
34. P-bromophenol [mi]
35. Schembl17443
36. P-bromophenol [hsdb]
37. Bidd:er0009
38. Dtxsid3051543
39. Chebi:47248
40. Nsc4970
41. 4-bromophenol, Analytical Standard
42. Zinc404316
43. Bcp17755
44. Str02366
45. Bdbm50150779
46. Stl281864
47. Akos000118965
48. Ps-3353
49. Db-023687
50. 4-bromophenol, Purum, >=98.0% (hplc)
51. Am20050178
52. B0787
53. B3910
54. Ft-0612047
55. En300-17967
56. 06b412
57. A801434
58. J-001587
59. J-514834
60. Q26421193
61. Z57127362
62. F0001-0119
| Molecular Weight | 173.01 g/mol |
|---|---|
| Molecular Formula | C6H5BrO |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 20.2 |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 66.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bromobenzene causes hepatic and extrahepatic toxicity in rats. Toxicity is related to the presence of covalently bound material in these tissues. A major bromobenzene metabolite, p-bromophenol, has been shown to give rise to covalently bound material in liver, lung and kidney in vivo, but is not toxic. p-Bromophenol is formed from bromobenzene in liver, lung and kidney microsomes and is subsequently metabolized to 4-bromocatechol and covalently bound material. Bromobenzene-3,4-oxide generated in situ by liver microsomes, is detoxified by kidney, liver and lung cytosol. The results suggest that the kidney toxicity caused by bromobenzene is probably not mediated by either bromobenzene-3,4-oxide or the reactive metabolites of p-bromophenol. In contrast, bromobenzene-3, 4-oxide may play a role in the lung toxicity observed after bromobenzene administration. However, the covalently bound material found in extrahepatic tissues may be derived from both bromobenzene-3,4-oxide or the reactive metabolites of p-bromophenol, which may be formed directly by these tissues or transported there from the liver.
PMID:6748863 Monks TJ, Lau SS; Life Sci 35 (5): 561-8 (1984)
4-Bromophenol and 4-bromocatechol are formed as metabolites of bromobenzene in vivo and in isolated rat hepatocytes. Both of these metabolites may potentially contribute to the hepatotoxicity of bromobenzene. Bromobenzene metabolism in hepatocytes isolated from phenobarbital-treated rats forms 0.12 to 0.17 mM 4-bromophenol and 4-bromocatechol in 2 hr, with 1 to 3 mM bromobenzene.
PMID:4002232 Dankovic DA, Billings RE; Toxicol Appl Pharmacol 79 (2): 323-31 (1985)
A microsomal metabolite of p-bromophenol was isolated and identified as 6-(glutathion-S-yl)-4-bromocatechol. p-Bromophenol is metabolized in rat liver microsomes in part to 4-bromocatechol. The catechol undergoes autooxidation to the corresponding quinone or semiquinone, which can either covalently bind to microsomal protein or, in the presence of glutathione, form a glutathione conjugate. Superoxide dismutase inhibited these reactions by preventing the superoxide anion-mediated oxidation of 4-bromocatechol. Thus, in the presence of glutathione, superoxide dismutase caused a decrease in conjugate formation with a corresponding increase in 4-bromocatechol levels. Conditions which increased the in vitro covalent binding of p-bromophenol (namely, phenobarbital treatment and the absence of glutathione) did not cause toxicity in vivo. Thus, chemically reactive metabolite(s) of p-bromophenol do not play a role in bromobenzene-mediated hepatotoxicity.
PMID:6148209 Monks TJ et al; J.Drug Metab Dispos 12 (4): 432-7 (1984)
The metabolism of bromobenzene has been examined in isolated hepatocytes and liver microsomes from phenobarbital-induced rats and in phenobarbital-induced rats in vivo. The metabolite profile produced upon incubation of isolated rat hepatocytes with bromobenzene differed with the hepatocyte concentration. At a low hepatocyte concentration (0.5 x 10+6 cells/mL), 4-bromophenol was the major metabolite, while at higher hepatocyte concentrations (2.0 and 5.0 x 10+6 cells/mL) bromobenzene-3,4-dihydrodiol was the major metabolite. 4-Bromophenol was the primary metabolite in incubations with rat liver microsomes. In vivo, 3- and 4-bromophenol were more predominant, with very little dihydrodiol formed. 4-Bromocatechol, a potentially toxic metabolite of bromobenzene, was formed in vivo as well as in isolated hepatocytes and microsomes. However, the mechanism of catechol formation differed, as determined by the retention of a deuterium label at the para position of bromobenzene. In microsomes, 4-bromophenol was the predominant precursor metabolite of 4-bromocatechol. In isolated hepatocytes, although the relative contribution of 4-bromophenol as the bromocatechol precursor differed with hepatocyte concentration, bromobenzene-3,4-dihydrodiol was the predominant precursor at all concentrations. In vivo, as in isolated hepatocytes, 4-bromocatechol was formed primarily via bromobenzene-3,4-dihydrodiol.
PMID:1974190 Miller NE et al; Drug Metab Dispos 18 (3): 304-8 (1990)
For more Metabolism/Metabolites (Complete) data for 4-BROMOPHENOL (6 total), please visit the HSDB record page.
4-Bromophenol has known human metabolites that include (2S,3S,4S,5R)-6-(4-bromophenoxy)-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
