
1. Butyl Carbitol
2. Diethylene Glycol Mono-n-butyl Ether
3. Diethylene Glycol Monobutyl Ether
1. 112-34-5
2. Butyldiglycol
3. Diethylene Glycol Monobutyl Ether
4. Butyl Carbitol
5. Diethylene Glycol Butyl Ether
6. Butoxydiglycol
7. Ethanol, 2-(2-butoxyethoxy)-
8. Butyl Diglycol
9. Butyl Dioxitol
10. Butyl Digol
11. Butoxyethoxyethanol
12. Bucb
13. Dowanol Db
14. Glycol Ether Db
15. Poly-solv Db
16. Jeffersol Db
17. Ektasolve Db
18. Butoxydiethylene Glycol
19. Diglycol Monobutyl Ether
20. O-butyl Diethylene Glycol
21. Diethylene Glycol Mono-n-butyl Ether
22. Butoxy Diethylene Glycol
23. Diethylene Glycol N-butyl Ether
24. Diethylene Gylcol Monobutyl Ether
25. Nsc 407762
26. Ethanol, 2,2'-oxybis-, Monobutyl Ether
27. Monobutyl Diethylene Glycol Ether
28. 9tb90iyc0e
29. 2-(2-butoxyethoxy)-ethanol
30. 2-(2-n-butoxyethoxy)ethanol
31. Nsc-407762
32. Dsstox_cid_1519
33. Dsstox_rid_76196
34. Dsstox_gsid_21519
35. Caswell No. 121b
36. Caswell No. 125h
37. N-butyl Carbitol
38. Diethylene Glycol Butyl Ether, >=99%
39. 3,6-dioxadecanol
40. Cas-112-34-5
41. Ccris 5321
42. Hsdb 333
43. 3,6-dioxa-1-decanol
44. Einecs 203-961-6
45. Unii-9tb90iyc0e
46. Epa Pesticide Chemical Code 011502
47. Brn 1739225
48. Butadigol
49. Ai3-01954
50. Butyl Di-icinol
51. Diethylene Db
52. Degbe
53. Ethanol 2-butoxyethoxy
54. Butyl Oxitol Glycol Ether
55. 2-(n-butoxyethoxy)ethanol
56. Ec 203-961-6
57. Schembl15619
58. 2-(2-butoxyethoxy);ethanol
59. Butoxydiglycol [inci]
60. Diethylene Glycol Butyl Ester
61. Diethyleneglycol Monobutylether
62. Diethyleneglycol N-butyl Ether
63. Wln: Q2o2o4
64. Diethyleneglycol Monobutyl Ether
65. Chembl1904721
66. Diethylene Glycol-monobutyl Ether
67. Dtxsid8021519
68. Zinc1600070
69. Tox21_202404
70. Tox21_300084
71. Ethanol,2'-oxybis-, Monobutyl Ether
72. Mfcd00002881
73. Nsc407762
74. Akos009156535
75. Diethylene Glycol Monobutyl Ether, 98%
76. Ncgc00164235-01
77. Ncgc00164235-02
78. Ncgc00164235-03
79. Ncgc00253937-01
80. Ncgc00259953-01
81. Ls-13547
82. B0699
83. Ft-0624889
84. Diethylene Glycol Monobutyl Ether [mi]
85. Diethylene Glycol Monobutyl Ether Reagent Grade
86. F71187
87. A802556
88. Diethylene Glycol Mono-n-butyl Ether [hsdb]
89. Diethylene Glycol Monobutyl Ether, >=98.0% (gc)
90. J-002756
91. J-519970
92. Q1018210
93. Diethylene Glycol Butyl Ether, Saj Special Grade, >=99.0%
94. Diethylene Glycol Monobutyl Ether, For Surfactant Analysis, >=99.0%
| Molecular Weight | 162.23 g/mol |
|---|---|
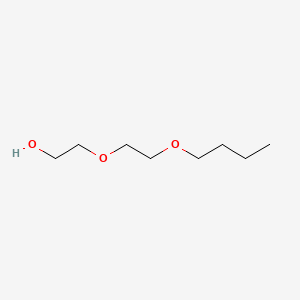
| Molecular Formula | C8H18O3 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 162.125594432 g/mol |
| Monoisotopic Mass | 162.125594432 g/mol |
| Topological Polar Surface Area | 38.7 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 66.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPTL THER/ Structure-activity studies with nine glycol alkyl ethers were conducted with a cellular leukemia transplant model in male Fischer rats to measure the effects on neoplastic progression in transplant recipients. Chemicals were given ad libitum in the drinking water simultaneously with the transplants and continued throughout the study. In all, 20 million leukemic cells were injected sc into syngeneic rats, which after 60 days resulted in a 10-fold increase in relative spleen weights, a 100-fold increase in white blood cell counts, and a 50% reduction in red blood cell indices and platelet counts. Ethylene glycol monomethyl ether given at a dose of 2.5 mg/mL in the drinking water completely eliminated all clinical, morphological, and histopathological evidence of leukemia, whereas the same dose of ethylene glycol monoethyl ether reduced these responses by about 50%. Seven of the glycol ethers were ineffective as anti-leukemic agents, including ethylene glycol, the monopropyl, monobutyl, and monophenyl ethylene glycol ethers, diethylene glycol, and the monomethyl and monoethyl diethylene glycol ethers. Ethylene glycol monomethyl ether more than doubled the latency period of leukemia expression and extended survival for at least 210 days. A minimal effective dose for a 50% reduction in the leukemic responses was 0.25 mg/mL ethylene glycol monomethyl ether in the drinking water (15 mg/kg body weight), whereas a 10-fold higher dose of 2-ethylene glycol monoethyl ether was required for equivalent antileukemic activity. In addition, the in vitro exposure of a leukemic spleen mononuclear cell culture to ethylene glycol monomethyl ether caused a dose- and time-dependent reduction in the number of leukemia cells after a single exposure to 1-100 mM concentrations, whereas the ethylene glycol monomethyl ether metabolite, 2-methoxyacetic acid, was only half as effective.
PMID:2357763 Dieter MP et al; Cancer Chemother Pharmacol 26 (3): 173-80 (1990)
Butyl carbitol can be absorbed through skin, but only in toxic amt if application is prolonged & continuous.
Browning, E. Toxicity and Metabolism of Industrial Solvents. New York: American Elsevier, 1965., p. 635
In vitro percutaneous absorption studies were carried out for ... /tritiated water, 2-ethoxyethyl acetate, diethylene glycol monobutyl ether, urea, di(2-ethylhexyl) phthalate, 2-ethylhexanol, ethyl 3-ethoxypropionate, and 2-propoxyethanol/ using full thickness rat skin and human stratum corneum. The purpose of the studies was to compare the rates of absorption for the two species. For each of the chemicals, the observed rate using full thickness rat skin was greater than that observed for human stratum corneum. The ratios of the rates (rat/human) varied from 1.7 to 5.8 with a mean value of 3.1. ... It was concluded that rat skin was more permeable than human skin for each of these eight chemicals. ...
PMID:1426706 Barber ED et al; Fundam Appl Toxicol 19 (4): 493-7 (1992)
Male and female Sprague-Dawley rats received 200 and 2000 mg neat (14)C-DEGBE/kg bw and 200 mg of a 10% aqueous solution/kg bw. Dermal adsorption rate was 0.73 and 1.46 mg/sq cm/hr for males and females respectively.
European Chemicals Bureau; IUCLID Dataset, 2-(2-butoxyethoxy) ethanol (CAS # 112-34-5) p.112 Available from, as of February 26, 2007: https://esis.jrc.ec.europa.eu/
To assist the evaluation of the hazards of skin contact with selected undiluted glycol ethers, their absorption across isolated human abdominal epidermis was measured in vitro. Epidermal membranes were set up in glass diffusion cells and, following an initial determination of permeability to tritiated water, excess undiluted glycol ether was applied to the outer surface for 8 hr. The appearance of glycol ether in an aqueous "receptor" phase bathing the underside of the epidermis was quantified by a gas chromatographic technique. A final determination of tritiated water permeability was compared with initial values to establish any irreversible alterations in epidermal barrier function induced by contact with the glycol ethers. 2-Methoxyethanol was most readily absorbed (mean steady rate 2.82 mg/sq cm/hr), and a relatively high absorption rate (1.17 mg/sq cm/hr) was also apparent for 1-methoxypropan-2-ol. There was a trend of reducing absorption rate with increasing molecular weight or reducing volatility for monoethylene glycol ethers (2-methoxyethanol, 2.82 mg/sq cm/hr; 2-ethoxyethanol, 0.796 mg/sq cm/hr; 2-butoxyethanol, 0.198 mg/sq cm/hr) and also within the diethylene glycol series: 2-(2-methoxyethoxy) ethanol (0.206 mg/sq cm/hr); 2-(2-ethoxyethoxy) ethanol (0.125 mg/sq cm/hr) and 2-(2-butoxyethoxy) ethanol (0.05 mg/sq cm/hr). The rate of absorption of 2-ethoxyethyl acetate was similar to that of the parent alcohol, 2-ethoxyethanol. Absorption rates of diethylene glycol ethers were slower than their corresponding monoethylene glycol equivalents. Combination of intrinsic toxicity and ability to pass across skin contribute to assessment of hazards of contact with undiluted glycol ethers.
PMID:6499804 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1568269 Dugard PH et al; Environ Health Perspect 57: 193-7 (1984)
For more Absorption, Distribution and Excretion (Complete) data for DIETHYLENE GLYCOL MONO-N-BUTYL ETHER (8 total), please visit the HSDB record page.
Male and female Sprague-Dawley rats received 200 and 2000 mg neat (14)C-DEGBE/kg bw and 200 mg of a 10% aqueous solution/kg bw. 2-(2-butoxyethanol)acetic acid was the major urinary metabolite identified and the glucuronide conjugate was present at levels of 5.2% to 8.2% of the urinary (14)C.
European Chemicals Bureau; IUCLID Dataset, 2-(2-butoxyethoxy) ethanol (CAS # 112-34-5) p.111 Available from, as of February 26, 2007: https://esis.jrc.ec.europa.eu/
The metabolism of diethylene glycol monobutyl ether acetate was studied in vitro and in vivo in male Sprague-Dawley rats. The extent of conversion of diethylene glycol monobutyl ether acetate to diethylene glycol monobutyl ether was determined. In vitro, diethylene glycol monobutyl ether acetate was rapidly hydrolyzed by rat blood to diethylene glycol monobutyl ether; the biological half-life was less than 3 min. The urine was the major pathway for eliminating diethylene glycol monobutyl ether acetate derived (14)C activity; 80% was eliminated after 24 hr. Only 2 to 3% of each dose was eliminated in the feces. About 5% of each dose was eliminated in the expired air, mostly as radioactive carbon dioxide. Only 1-3% doses were found in the tissues and ied. No unchanged diethylene glycol monobutyl ether acetate or diethylene glycol monobutyl ether or 2-butoxyacetic acid, a putative hematotoxic diethylene glycol monobutyl ether acetate metabolite, was found. Diethylene glycol monobutyl ether acetate is rapidly hydrolyzed to diethylene glycol monobutyl ether by rat blood. The biological effects of diethylene glycol monobutyl ether acetate and diethylene glycol monobutyl ether would be indistinguishable. No 2-butoxyacetic acid is produced.
PMID:2815838 Deisinger PJ, Guest D; Xenobiotica 19 (9): 981-9 (1989)
In the present study, floor lacquerers' (n = 22) inhalation and total exposure to 2-(2-alkoxy)ethoxyethanols was measured. The measurements of inhalation exposure were done with charcoal tubes, and total exposure was biomonitored by urinalysis of 2-(2-alkoxyethoxy)acetic acids. The 8hr inhalation exposures of floor lacquerers to 2-(2-methoxyethoxy)ethanol (DEGME), 2-(2-ethoxyethoxy)ethanol (DEGEE) and 2-(2-butoxyethoxy)ethanol (DEGBE) were in average 0.23 +/- 0.07 ppm (average+/-S.D., n = 3), 0.08 +/- 0.07 ppm (n = 16), and 0.05 +/- 0.03 ppm (n = 16), respectively. The excretions of 2-(2-methoxyethoxy)acetic acid (MEAA), 2-(2-ethoxyethoxy)acetic acid (EEAA) and 2-(2-butoxyethoxy)acetic acid (BEAA) were in average 4.9 +/- 4.3 mmol/mol creatinine, 9.3 +/- 8.0 mmol/mol creatinine and 9.2 +/- 7.4 mmol/mol creatinine, respectively. A linear relationship was found between the urinary 2-(2-alkoxyethoxy)acetic acid concentrations and the preceding 8-hr occupational exposure to 2-(2-alkoxyethoxy)ethanol. /2-(2-Alkoxyethoxy)ethanol/
PMID:15705492 Laitinen J, Pulkkinen J; Toxicol Lett 156 (1): 117-26 (2005)