


1. (13c3)-gs-441524
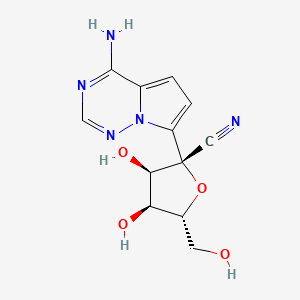
2. (2r,3r,4s,5r)-2-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydro-2-furancarbonitrile
3. 13c3 Gs-441524
4. 13c3-gs-441524
5. 2-c-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2,5-anhydro-d-altrononitrile
6. Gs 441524
7. Gs-441285
8. Gs-441524
9. Gs-828840
10. Gs441524
1. Gs-441524
2. 1191237-69-0
3. Gs441524
4. Gs 441524
5. Evo984
6. (2r,3r,4s,5r)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)oxolane-2-carbonitrile
7. Evo-984
8. 1bqk176dt6
9. Evo 984
10. 2-c-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2,5-anhydro-d-altrononitrile
11. 2-c-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2,5-anhydro-d-altrononitrile
12. D-altrononitrile, 2-c-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-2,5-anhydro-
13. (2~{r},3~{r},4~{s},5~{r})-2-(4-azanylpyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-(hydroxymethyl)-3,4-bis(oxidanyl)oxolane-2-carbonitrile
14. Reacted Form
15. Mfcd32666994
16. (2r,3r,4s,5r)-2-{4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl}-3,4-dihydroxy-5-(hydroxymethyl)oxolane-2-carbonitrile
17. U08
18. Unii-1bqk176dt6
19. Chembl2016757
20. Schembl10120689
21. Gtpl11445
22. Chebi:147281
23. Dtxsid201028047
24. Ex-a3056
25. Remdesivir Metabolite(gs-441524)
26. Bdbm50571531
27. S6814
28. Zb1788
29. Gs 441524 [who-dd]
30. Akos037648588
31. Remdesivir Intermediate(gs-441524)
32. Bcp29949-1
33. (2r,3r,4s,5r)-2-(4-aminopyrrolo(2,1-f)(1,2,4)triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydro-2-furancarbonitrile
34. Ac-31365
35. Bs-14744
36. Hy-103586
37. Cs-0028160
38. C22275
39. D78523
40. A933921
41. (2r,3r,4s,5r)-2-(4-aminopyrrolo[1,2-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile
| Molecular Weight | 291.26 g/mol |
|---|---|
| Molecular Formula | C12H13N5O4 |
| XLogP3 | -1.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 291.09675391 g/mol |
| Monoisotopic Mass | 291.09675391 g/mol |
| Topological Polar Surface Area | 150 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 456 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
Absorption
GS-441524 has been found to transport poorly into cells compared to remdesivir.
GS-441524 is phosphorylated 3 times to form the active nucleoside triphosphate.
GS-441524 is phosphorylated 3 times to form the active nucleoside triphosphate, which is incorporated into the genome of virions, terminating its replication.
