

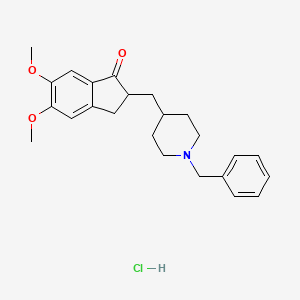

1. 1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine Hydrochloride
2. Aricept
3. Donepezil
4. Donepezilium Oxalate Trihydrate
5. E 2020
6. E-2020
7. E2020
8. Eranz
1. 120011-70-3
2. Donepezil Hcl
3. Aricept
4. Bnag
5. 110119-84-1
6. 2-((1-benzylpiperidin-4-yl)methyl)-5,6-dimethoxy-2,3-dihydro-1h-inden-1-one Hydrochloride
7. E2020
8. Donepezilhcl
9. E-2020
10. Aricept Odt
11. E 2020
12. Donepezil (hydrochloride)
13. Donepezil, Hcl
14. 2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1h-inden-1-one Hydrochloride
15. Nsc-737535
16. Nsc-758882
17. Chembl1678
18. 3o2t2pj89d
19. 2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydroinden-1-one;hydrochloride
20. 2-(1-benzyl-4-piperidylmethyl)-5,6-dimethoxy-1-indanone Hydrochloride
21. 120011-70-3 (hcl)
22. E2020;e-2020
23. Dsstox_cid_26698
24. Dsstox_rid_81832
25. Dsstox_gsid_46698
26. 1h-inden-1-one, 2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-, Hydrochloride (1:1)
27. Eranz
28. 2-(1-benzyl-piperidin-4-ylmethyl)-5,6-dimethoxy-indan-1-one Hydrochloride
29. E 2020 (pharmaceutical)
30. Smr000449292
31. Cas-120011-70-3
32. Donepezil Hydrochloride [usan]
33. Nsc 737535
34. Nsc 758882
35. Ncgc00167537-01
36. Unii-3o2t2pj89d
37. Memorit
38. Memac
39. Aricept Evess
40. Donepezil,hcl
41. Aricept D
42. (+/-)-2-((1-benzyl-4-piperidyl)methyl)-5,6-dimethoxy-1-indanone Hydrochloride
43. (+/-)-2-[(1-benzyl-4-piperidyl)methyl]-5,6-dimethoxy-1-indanone Hydrochloride
44. Aricept (tn)
45. Donepezil Hydrochloride [usan:usp]
46. Adlarity
47. Donepezil, Hydrochloride
48. 1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine Hcl
49. Donepezil Hcl (aricept)
50. Donepezil Hcl - Bio-x
51. Schembl1815
52. Donepezil Hydrochloride,(s)
53. Mls000758276
54. Mls001401387
55. Chebi:4696
56. Dtxsid0046698
57. Pharmakon1600-01504403
58. Act02683
59. Amy40527
60. Bcp02205
61. Bcp24884
62. Hy-b0034
63. Donepezil Hydrochloride [mi]
64. Tox21_112534
65. Ac-913
66. Donepezil Hydrochloride (jp17/usp)
67. Donepezil Hydrochloride [jan]
68. Mfcd00881312
69. Nsc737535
70. Nsc758882
71. S2462
72. Akos000277343
73. Akos016340656
74. Tox21_112534_1
75. Ccg-101043
76. Ccg-213309
77. Cs-0863
78. Donepezil Hydrochloride [mart.]
79. Donepezil Hydrochloride [vandf]
80. Ks-1051
81. Nc00293
82. (+-)-2-((1-benzyl-4-piperidyl)methyl)-5,6-dimethoxy-1-indanone Hydrochloride
83. Donepezil Hydrochloride [usp-rs]
84. Donepezil Hydrochloride [who-dd]
85. Ncgc00167537-02
86. 1h-inden-1-one, 2,3-dihydro-5,6-dimethoxy-2-((1-(phenylmethyl)-4-piperidinyl)methyl)-, Hydrochloride
87. Bd164380
88. D4099
89. Donepezil Hydrochloride [orange Book]
90. Ft-0602354
91. Ft-0625589
92. Donepezil Hydrochloride [ep Monograph]
93. D00670
94. Donepezil Hydrochloride [usp Monograph]
95. E-2022
96. Namzaric Component Donepezil Hydrochloride
97. 119d841
98. A804411
99. Donepezil Hydrochloride Component Of Namzaric
100. Donepezil Hydrochloride Monohydrate, >=98% (hplc)
101. Q-100096
102. Q-201040
103. Q27106438
104. Donepezil Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
105. 2,3-dihydro-5,6-dimethoxy-2-((1-(phenylmethyl)-4-piperidinyl)methyl)-1h-inden-1-one Hcl
106. 2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]-1h-inden-1-one
107. (+-)-2,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1h-inden-1-one Hydrochloride
108. 2-((1-benzylpiperidin-4-yl)methyl)-5,6-dimethoxy-2,3-dihydro-1h-inden-1-one Hydrochloride 120014-06-
| Molecular Weight | 416.0 g/mol |
|---|---|
| Molecular Formula | C24H30ClNO3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 415.1914215 g/mol |
| Monoisotopic Mass | 415.1914215 g/mol |
| Topological Polar Surface Area | 38.8 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 510 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Nootropic Agents
Drugs used to specifically facilitate learning or memory, particularly to prevent the cognitive deficits associated with dementias. These drugs act by a variety of mechanisms. (See all compounds classified as Nootropic Agents.)