
1. Clopidogrel
2. Clopidogrel Besilate
3. Clopidogrel Besylate
4. Clopidogrel Hydrochloride
5. Clopidogrel Mepha
6. Clopidogrel Napadisilate
7. Clopidogrel Sandoz
8. Clopidogrel, (+)(s)-isomer
9. Clopidogrel-mepha
10. Iscover
11. Pcr 4099
12. Pcr-4099
13. Plavix
14. Sc 25989c
15. Sc 25990c
16. Sr 25989
1. 120202-66-6
2. Clopidogrel Sulfate
3. Clopidogrel Hydrogen Sulfate
4. Iscover
5. Plavix
6. Clopidogrel Bisulphate
7. Clopidogrel Hemisulfate
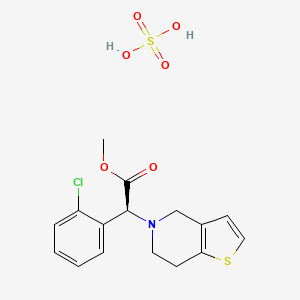
8. (s)-methyl 2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4h)-yl)acetate Sulfate
9. Clopidogrel Hydrogen Sulphate
10. Clopidogrel Bms
11. Clopidogrel Sulphate
12. Sr 25990c
13. Clopidogrel (hydrogen Sulfate)
14. Isocover
15. Pidogrel
16. Clopidogrel-bgr
17. Clopidogrel-bms
18. Clopidogrel Teva
19. Clopidogrel Zentiva
20. Clopidogrel (plavix)
21. Clopidogrel Ratiopharm
22. Sr-25990c
23. S-(+)-clopidogrel Hydrogen Sulfate
24. Clopidogrel (as Bisulfate)
25. (s)-(+)-clopidogrel Sulfate
26. 08i79htp27
27. 120202-66-6 (sulfate)
28. Pm-103
29. Methyl (s)-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4h)-yl)acetate Sulfate
30. 144077-07-6
31. Myogrel
32. Clopidogrel Hydrogensulfate
33. Plavix (tn)
34. Dsstox_cid_26024
35. Dsstox_rid_81297
36. Dsstox_gsid_46024
37. Plavitor
38. Osvix
39. Chebi:3759
40. Chembl1083385
41. Methyl (2s)-2-(2-chlorophenyl)-2-(6,7-dihydro-4h-thieno[3,2-c]pyridin-5-yl)acetate Sulfate
42. Methyl (2s)-2-(2-chlorophenyl)-2-(6,7-dihydro-4h-thieno[3,2-c]pyridin-5-yl)acetate;sulfuric Acid
43. Sulfuric Acid Methyl (2s)-2-(2-chlorophenyl)-2-{4h,5h,6h,7h-thieno[3,2-c]pyridin-5-yl}acetate
44. Clopidogrel Bisulfate [usan]
45. Cas-135046-48-9
46. Unii-08i79htp27
47. R-(-)-clopidogrel Hydrogen Sulfate
48. Clopidogrel Bisulfate [usan:usp]
49. Isocover (tn)
50. Clopidogrel Bisolfato
51. (s)-(+)-clopidogrel Hydrogen Sulfate
52. Bisulfato De Clopidogrel
53. Bissulfato De Clopidogrel
54. Ncgc00159321-02
55. (+)-clopidogrel Bisulfate
56. (+) Clopidogrel Bisulfate
57. Methyl Alphas-(2-chlorophenyl)-6,7-dihydrothieno(3,2-c)pyridine-5(4h)-acetate Sulfate (1:1)
58. Clopidogrel Bisulfate (usp)
59. Clopidogrel Sulfate (jp17)
60. Schembl33556
61. Clopidogrel Hydrogen Sulphate
62. Spectrum1503710
63. Mdco-157
64. (+)-clopidogrel Hydrogen Sulfate
65. Clopidogrel Sulfate [jan]
66. Dtxsid601016080
67. Hms1922g16
68. Hms2093i13
69. Hms3884g15
70. Pharmakon1600-01503710
71. Amy40591
72. Clopidogrel Sulfate Tablets (jp17)
73. Tox21_111570
74. Ccg-39568
75. Clopidogrel Bisulfate [vandf]
76. Mfcd00876395
77. Nsc758613
78. S1415
79. Clopidogrel Bisulfate [mart.]
80. Akos015900408
81. Clopidogrel Bisulfate [usp-rs]
82. Clopidogrel Bisulfate [who-dd]
83. Tox21_111570_1
84. Ac-2135
85. Cs-1882
86. Dv-7314
87. Ks-1045
88. S65c842
89. Clopidogrel (as Hydrogen Sulfate)
90. Clopidogrel Hydrogen Sulfate [mi]
91. Ncgc00163329-03
92. 111ge004
93. Bc164326
94. Hy-17459
95. Methyl (+)-(s)-alpha-(o-chlorophenyl)-6,7-dihydrothieno(3,2-c)pyridine-5(4h)-acetate, Sulfate (1:1)
96. Thieno(3,2-c)pyridine-5(4h)-acetic Acid, 6,7-dihydro-alpha-(2-chlorophenyl)-, Methyl Ester, (s)-, Sulfate
97. Thieno(3,2-c)pyridine-5(4h)-acetic Acid, Alpha-(2-chlorophenyl)-6,7-dihydro-, Methyl Ester, (alphas)-, Sulfate (1:1)
98. Thieno(3,2-c)pyridine-5(4h)-acetic Acid, Alpha-(2-chlorophenyl)-6,7-dihydro-, Methyl Ester, (s)-, Sulfate (1:1)
99. Thieno[3,2-c]pyridine-5(4h)-acetic Acid,a-(2-chlorophenyl)-6,7-dihydro-, Methyl Ester, (as)-, Sulfate (1:1)
100. Clopidogrel Bisulfate [orange Book]
101. Clopidogrel Bisulfate [usp Monograph]
102. Sw219457-1
103. Clopidogrel Hydrogen Sulfate [ema Epar]
104. D00769
105. Duocover Component Clopidogrel Bisulfate
106. Duoplavin Component Clopidogrel Bisulfate
107. Clopidogrel Hydrogen Sulphate [ema Epar]
108. Clopidogrel Bisulfate Component Of Duocover
109. Clopidogrel Bisulfate Component Of Duoplavin
110. Clopidogrel Hydrogen Sulfate [ep Monograph]
111. Sr-05000002068
112. J-006637
113. Sr-05000002068-1
114. (+)-clopidogrel1r(-)camphor-10-sulphonic Acid Salt
115. (s)-(+)-clopidogrel Hydrogensulfate, >=98% (hplc)
116. Q27888063
117. Z1550648767
118. (s)-(+)-clopidogrel Bisulfate;(s)-(+)-clopidogrel Hydrogen Sulfate
119. Clopidogrel Bisulfate, United States Pharmacopeia (usp) Reference Standard
120. Clopidogrel Hydrogen Sulfate, European Pharmacopoeia (ep) Reference Standard
121. Clopidogrel/acetylsalicyclic Acid Teva Component Clopidogrel Bisulfate
122. (s)-(+)-methyl (2-chlorophenyl)(6,7-dihydro-4h-thieno(3,2-c)pyrid-5-yl)acetate Bisulfate
123. Clopidogrel Bisulfate Component Of Clopidogrel/acetylsalicyclic Acid Teva
124. Clopidogrel Bisulfate, Pharmaceutical Secondary Standard; Certified Reference Material
125. Clopidogrel For System Suitability, European Pharmacopoeia (ep) Reference Standard
126. Methyl(s)-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-c]pyridin-5(4h)-yl)acetatesulfate
127. (s)-(+)-methyl 2-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)-2-(2-chlorophenyl)acetate Hydrogen Sulfate
128. (s)-(2-chloro-phenyl)-(6,7-dihydro-4h-thieno[3,2-c]pyridin-5-yl)-acetic Acid Methyl Ester Sulfuric Acid Salt
129. (s)-methyl-(2-chloro-phenyl)-(6,7-dihydro-4h-thieno[3,2-c]pyridin-5-yl)-acetate H2so4
130. Methyl (+)-(s)-.alpha.-(o-chlorophenyl)-6,7-dihydrothieno(3,2-c)pyridine-5(4h)-acetate, Sulfate (1:1)
131. Methyl (+)-(s)-.alpha.-(o-chlorophenyl)-6,7-dihydrothieno(3,2-c)pyridine-5(4h)-acetate, Sulphate (1:1)
132. Methyl (2s)-2-(2-chlorophenyl)-2-{4h,5h,6h,7h-thieno[3,2-c]pyridin-5-yl}acetate; Sulfuric Acid
133. Methyl (s)-(+)-2-(2-chlorophenyl)-2-[6,7-dihydro-4h-thieno[3,2-c]pyridin-5-yl]acetate Sulfate
134. Thieno(3,2-c)pyridine-5(4h)-acetic Acid, .alpha.-(2-chlorophenyl)-6,7-dihydro-, Methyl Ester, (s)-, Sulfate (1:1)
135. Thieno(3,2-c)pyridine-5(4h)-acetic Acid, .alpha.-(2-chlorophenyl)-6,7-dihydro-, Methyl Ester, (s)-, Sulphate (1:1)
| Molecular Weight | 419.9 g/mol |
|---|---|
| Molecular Formula | C16H18ClNO6S2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 419.0264073 g/mol |
| Monoisotopic Mass | 419.0264073 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 463 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | CLOPIDOGREL BISULFATE |
| Active Ingredient | CLOPIDOGREL BISULFATE |
| Company | ACCORD HLTHCARE (Application Number: A202925); ACME LABS (Application Number: A078004); AMNEAL PHARMS (Application Number: A203751); APOTEX INC (Application Number: A076274); AUROBINDO PHARMA LTD (Application Number: A090540); CSPC OUYI PHARM CO (Application Number: A204359); DR REDDYS LABS INC (Application Number: A076273); DR REDDYS LABS LTD (Application Number: A091023); GATE PHARMS (Application Number: A091216); MACLEODS PHARMS LTD (Application Number: A202928); MYLAN PHARMS INC (Application Number: A077665); SCIEGEN PHARMS INC (Application Number: A204165); SUN PHARM INDUSTRIES (Application Number: A078133); SUN PHARMA GLOBAL (Application Number: A090494); TEVA PHARMS (Application Number: A090625); TEVA (Application Number: A076999); TORRENT PHARMS LTD (Application Number: A090844); WOCKHARDT LTD (Application Number: A202266); ZYDUS PHARMS USA INC (Application Number: A201686) |
| 2 of 2 | |
|---|---|
| Drug Name | PLAVIX |
| Active Ingredient | CLOPIDOGREL BISULFATE |
| Company | SANOFI AVENTIS US (Application Number: N020839. Patents: 6429210, 6429210*PED, 6504030, 6504030*PED) |
Secondary prevention of atherothrombotic events Clopidogrel is indicated in:
- Adult patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
- Adult patients suffering from acute coronary syndrome:
- Non-ST segment elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
- ST segment elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
Prevention of atherothrombotic and thromboembolic events in atrial fibrillation
In adult patients with atrial fibrillation who have at least one risk factor for vascular events, are not suitable for treatment with Vitamin K antagonists (VKA) and who have a low bleeding risk, clopidogrel is indicated in combination with ASA for the prevention of atherothrombotic and thromboembolic events, including stroke.
Secondary prevention of atherothrombotic events
Clopidogrel is indicated in:
- Adult patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
- Adult patients suffering from acute coronary syndrome:
- Non-ST segment elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
- ST segment elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
- Prevention of atherothrombotic and thromboembolic events in atrial fibrillation
In adult patients with atrial fibrillation who have at least one risk factor for vascular events, are not suitable for treatment with Vitamin K antagonists (VKA) and who have a low bleeding risk, clopidogrel is indicated in combination with ASA for the prevention of atherothrombotic and thromboembolic events, including stroke.
* Secondary prevention of atherothrombotic events:
Clopidogrel is indicated in:
- Adult patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
- Adult patients suffering from acute coronary syndrome:
- Non-ST segment elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
- ST segment elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
In patients with moderate to high-risk Transient Ischemic Attack (TIA) or minor Ischemic Stroke (IS)
Clopidogrel in combination with ASA is indicated in:
- Adult patients with moderate to high-risk TIA (ABCD2 score 4) or minor IS (NIHSS 3) within 24 hours of either the TIA or IS event.
* Prevention of atherothrombotic and thromboembolic events in atrial fibrillation:
In adult patients with atrial fibrillation who have at least one risk factor for vascular events, are not suitable for treatment with Vitamin K antagonists (VKA) and who have a low bleeding risk, clopidogrel is indicated in combination with ASA for the prevention of atherothrombotic and thromboembolic events, including stroke.
Prevention of atherothrombotic events Clopidogrel is indicated in:
Adult patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
Prevention Secondary prevention of atherothrombotic events Clopidogrel is indicated in:
- Adult patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
- Adult patients suffering from acute coronary syndrome:
- Non-ST segment elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
- ST segment elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
- Prevention of atherothrombotic and thromboembolic events in atrial fibrillation:
- In adult patients with atrial fibrillation who have at least one risk factor for vascular events, are not suitable for treatment with Vitamin K antagonists (VKA) and who have a low bleeding risk, clopidogrel is indicated in combination with ASA for the prevention of atherothrombotic and thromboembolic events, including stroke.
* Secondary prevention of atherothrombotic events:
Clopidogrel is indicated in:
- adult patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from seven days until less than six months) or established peripheral arterial disease;
- adult patients suffering from acute coronary syndrome:
- non-ST-segment-elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA);
- ST-segment-elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
- In patients with moderate to high-risk Transient Ischemic Attack (TIA) or minor Ischemic Stroke (IS)
Clopidogrel in combination with ASA is indicated in:
- Adult patients with moderate to high-risk TIA (ABCD2 score 4) or minor IS (NIHSS 3) within 24 hours of either the TIA or IS event.
* Prevention of atherothrombotic and thromboembolic events in atrial fibrillation:
In adult patients with atrial fibrillation who have at least one risk factor for vascular events, are not suitable for treatment with vitamin-K antagonists and who have a low bleeding risk, clopidogrel is indicated in combination with ASA for the prevention of atherothrombotic and thromboembolic events, including stroke.
Clopidogrel is indicated in adults for the prevention of atherothrombotic events in:
- patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
For further information please refer to section 5. 1.
Clopidogrel is indicated in adults for the prevention of atherothrombotic events in:
- Patients suffering from myocardial infarction (from a few days until less than 35 days), ischaemic stroke (from 7 days until less than 6 months) or established peripheral arterial disease.
- Patients suffering from acute coronary syndrome:
Non-ST segment elevation acute coronary syndrome (unstable angina or non-Q-wave myocardial infarction), including patients undergoing a stent placement following percutaneous coronary intervention, in combination with acetylsalicylic acid (ASA).
ST segment elevation acute myocardial infarction, in combination with ASA in medically treated patients eligible for thrombolytic therapy.
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Purinergic P2Y Receptor Antagonists
Compounds that bind to and block the stimulation of PURINERGIC P2Y RECEPTORS. Included under this heading are antagonists for specific P2Y receptor subtypes. (See all compounds classified as Purinergic P2Y Receptor Antagonists.)
B01AC04
B01AC04
B01AC04
B01AC03
B01AC04
B01AC04
B01AC04
B01AC04