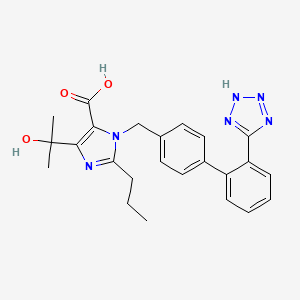

1. 4-(hydroxy-1-methylethyl)-2-propyl-1-((2'-(1h-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl)-1h-imidazole-5-carboxylic Acid
2. Cs-088
3. Omesartan
4. Rnh 6270
1. 144689-24-7
2. Olmesartan Acid
3. Rnh-6270
4. Rnh 6270
5. Cs-088
6. 1-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylic Acid
7. Nsc-759810
8. Chembl1516
9. 8w1iqp3u10
10. Chebi:48416
11. 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylic Acid
12. Votum
13. 1h-imidazole-5-carboxylic Acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-((2'-(1h-tetrazol-5-yl) (1,1'-biphenyl)-4-yl)methyl)-
14. 4-(2-hydroxypropan-2-yl)-2-propyl-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
15. Olmesartan [usan]
16. Omesartan
17. Olme Sartan
18. 1-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylic Acid
19. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]-1h-imidazole-5-carboxylic Acid (olmesartan)
20. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
21. 4-(hydroxy-1-methylethyl)-2-propyl-1-((2'-(1h-tetrazol-5-yl)-1,1'-biphenyl-4-yl)methyl)-1h-imidazole-5-carboxylic Acid
22. 4-(hydroxy-1-methylethyl)-2-propyl-1-{[2'-(1h-tetrazol-5-yl)-1,1'-biphenyl-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
23. Smr000466337
24. Unii-8w1iqp3u10
25. Olmesartan [usan:inn:ban]
26. Benicar;olmetec
27. Hsdb 8214
28. 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylic Acid
29. Olmesartan Medoximil
30. Cs 088
31. Diclofenacdiethylamine
32. Mfcd00914967
33. Olmesartan [mi]
34. Olmesartan [inn]
35. Olmesartan (usan/inn)
36. Olmesartan [vandf]
37. Ec 646-413-5
38. Olmesartan [who-dd]
39. Schembl94037
40. 1h-imidazole-5-carboxylic Acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-
41. Mls000759446
42. Mls001424016
43. Mls006011945
44. Dtxsid2040571
45. Olmesartan, >=98% (hplc)
46. Hms2051k12
47. Hms2235o24
48. Hms3369i09
49. Hms3393k12
50. Hms3604j06
51. Pharmakon1600-01505206
52. Zinc538621
53. Bcp12007
54. Bdbm50241364
55. De-092
56. Nsc759810
57. S5581
58. Akos015900241
59. Akos024458255
60. Ac-9385
61. Ccg-100868
62. Ccg-269198
63. Cs-0576
64. Db00275
65. Nc00118
66. Nsc 759810
67. Ncgc00246968-01
68. Ncgc00246968-02
69. Ncgc00246968-04
70. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1h-imidazole-5-carboxylic Acid
71. 4-(2-hydroxypropan-2-yl)-2-propyl-1-({4-[2-(1h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1h-imidazole-5-carboxylic Acid
72. As-10242
73. Hy-17004
74. Olmesartan Medoxomil Impurity, Olmesartan-
75. Smr004703526
76. Db-042742
77. Ft-0631169
78. O-125
79. O0507
80. C21543
81. D05246
82. 689o247
83. A853148
84. L001097
85. Olmesartan 100 Microg/ml In Acetonitrile:methanol
86. Q421156
87. W-201270
88. Olmesartan Medoxomil Impurity A [ep Impurity]
89. Z1583819783
90. Olmesartan Medoxomil Impurity, Olmesartan- [usp Impurity]
91. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-((2'-(2h-tetrazol-5-yl)biphenyl-4-yl)methyl)-1h-imidazole-5-carboxylate
92. 1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylic Acid
93. 1h-imidazole-5-carboxylicacid,4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-
94. 4-(1-hydroxy-1-methylethyl) -2-propyl-1-{4-[2-(tetrazole -5-yl)phenyl]phenyl}methylimidazole-5-carboxylic Acid
95. 4-(1-hydroxy-1-methylethyl) -2-propyl-1-{4-[2-(tetrazole-5-yl)phenyl]phenyl}methylimidazole-5-carboxylic Acid
96. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2''-(1h-tetrazol-5-yl)[1,1''-biphenyl]-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
97. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2''-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
98. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2'-(1h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
99. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(tetrazol-5-yl)phenyl]phenyl}methylimidazole-5-carboxylic Acid
100. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(tetrazole-5-yl)phenyl]phenyl}methylimidazole-5-carboxylic Acid
101. 4-(2-hydroxypropan-2-yl)-2-propyl-1-{[2'-(1h-1,2,3,4-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
102. 4-(hydroxy-1-methylethyl)-2-propyl-1-{[2''-(1h-tetrazol-5-yl)-1,1''-biphenyl-4-yl]methyl}-1h-imidazole-5-carboxylic Acid
103. 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylic Acid.
104. Olm
| Molecular Weight | 446.5 g/mol |
|---|---|
| Molecular Formula | C24H26N6O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 446.20663871 g/mol |
| Monoisotopic Mass | 446.20663871 g/mol |
| Topological Polar Surface Area | 130 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 656 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Angiotensin II Type 1 Receptor Blockers; Antihypertensive Agents
National Library of Medicine's Medical Subject Headings. Olmesartan. Online file (MeSH, 2014). Available from, as of September 2, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Benicar is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Benicar. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. It may be used alone or in combination with other antihypertensive agents. /Included in US product label/
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
Both angiotensin II receptor antagonists /including olmesartan/ and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2069
Angiotensin II receptor antagonists /inlcuding olmesartan/ have been used with equivocal results in the management of congestive heart failure. While angiotensin II receptor antagonists appear to share the hemodynamic effects of ACE inhibitors, some clinicians state that in the absence of data documenting comparable long-term cardiovascular and/or renal benefits, angiotensin II receptor antagonists should be reserved principally for patients in whom ACE inhibitors are indicated but who are unable to tolerate the drugs (e.g., because of cough). /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2069
The antihypertensive effects of Benicar in the pediatric population were evaluated in a randomized, double-blind study involving 302 hypertensive patients aged 6 to 16 years. The study population consisted of an all black cohort of 112 patients and a mixed racial cohort of 190 patients, including 38 blacks. The etiology of the hypertension was predominantly essential hypertension (87% of the black cohort and 67% of the mixed cohort). Patients who weighed 20 to <35 kg were randomized to 2.5 or 20 mg of Benicar once daily and patients who weighed > or = 35 kg were randomized to 5 or 40 mg of Benicar once daily. At the end of 3 weeks, patients were re-randomized to continuing Benicar or to taking placebo for up to 2 weeks. During the initial dose-response phase, Benicar significantly reduced both systolic and diastolic blood pressure in a weight-adjusted dose-dependent manner. Overall, the two dose levels of Benicar (low and high) significantly reduced systolic blood pressure by 6.6 and 11.9 mm Hg from the baseline, respectively. These reductions in systolic blood pressure included both drug and placebo effect. During the randomized withdrawal to placebo phase, mean systolic blood pressure at trough was 3.2 mm Hg lower and mean diastolic blood pressure at trough was 2.8 mm Hg lower in patients continuing Benicar than in patients withdrawn to placebo. These differences were statistically different. As observed in adult populations, the blood pressure reductions were smaller in black patients. In the same study, 59 patients aged 1 to 5 years who weighed > or = 5 kg received 0.3 mg/kg of Benicar once daily for three weeks in an open label phase and then were randomized to receiving Benicar or placebo in a double-blind phase. At the end of the second week of withdrawal, the mean systolic/diastolic blood pressure at trough was 3/3 mm Hg lower in the group randomized to Benicar; this difference in blood pressure was not statistically significant (95% C.I. -2 to 7/-1 to 7).
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Benicar as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Benicar as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the reninangiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Benicar, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
Neonates with a history of in utero exposure to Benicar: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
For more Drug Warnings (Complete) data for Olmesartan (19 total), please visit the HSDB record page.
Olmesartan is indicated for the treatment of hypertension either alone or in combination with other antihypertensive agents. Olmesartan is also used off-label for the management Type 2 Diabetes-associated nephropathy, heart failure, and post-myocardial infarction, particularly in patients who are unable to tolerate ACE inhibitors. ARBs such as olmesartan have been shown in a number of large-scale clinical outcomes trials to improve cardiovascular outcomes including reducing risk of myocardial infarction, stroke, the progression of heart failure, and hospitalization. Like other ARBs, olmesartan blockade of RAAS slows the progression of diabetic nephropathy due to its renoprotective effects.
FDA Label
Overall, olmesartan's physiologic effects lead to reduced blood pressure, lower aldosterone levels, reduced cardiac activity, and increased excretion of sodium. **Hypotension in Volume- or Salt-Depleted Patients** In patients with an activated renin-angiotensin aldosterone system, such as volume-and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), symptomatic hypotension may be anticipated after initiation of treatment with olmesartan. Initiate treatment under close medical supervision. If hypotension does occur, place the patient in the supine position and, if necessary, give an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized. Valvular Stenosis: there is concern on theoretical grounds that patients with aortic stenosis might be at a particular risk of decreased coronary perfusion, because they do not develop as much afterload reduction. **Impaired Renal Function** As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function may be anticipated in susceptible individuals treated with olmesartan. In patients whose renal function may depend upon the activity of the renin-angiotensin- aldosterone system (e.g., patients with severe congestive heart failure), treatment with angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor antagonists has been associated with oliguria and/or progressive azotemia and rarely with acute renal failure and/or death. Similar results may be anticipated in patients treated with olmesartan. In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen (BUN) have been reported. There has been no long-term use of olmesartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected. **Sprue-like Enteropathy** Severe, chronic diarrhea with substantial weight loss has been reported in patients taking olmesartan months to years after drug initiation. Intestinal biopsies of patients often demonstrated villous atrophy. If a patient develops these symptoms during treatment with olmesartan, exclude other etiologies. Consider discontinuation of olmesartan medoxomil in cases where no other etiology is identified. **Electrolyte Imbalances** Olmesartan medoxomil contains olmesartan, a drug that inhibits the renin-angiotensin system (RAS). Drugs that inhibit the RAS can cause hyperkalemia. Monitor serum electrolytes periodically.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
Absorption
When taken orally, the prodrug olmesartan medoxomil is rapidly absorbed in the gastrointestinal tract and metabolized to olmesartan. The esterification with medoxomil was created with the intention of increasing olmesartan bioavailability from 4.5% to 28.6%. Oral administration of 10-160 mg of olmesartan has been shown to reach peak plasma concentration of 0.22-2.1 mg/L after 1-3 hours with an AUC of 1.6-19.9mgh/L. The pharmacokinetic profile of olmesartan has been observed to be nearly linear and dose-dependent under the therapeutic range. The steady-state level of olmesartan is achieved after once a day dosing during 3 to 5 days.
Route of Elimination
The main elimination route of olmesartan is in the unchanged form through the feces. From the systemically bioavailable dose, about 10-16% is eliminated in the urine.
Volume of Distribution
17 L
Clearance
Total plasma clearance is 1.3 L/h and the renal clearance is 0.6 L/h.
In rats, olmesartan crossed the blood-brain barrier poorly, if at all. Olmesartan passed across the placental barrier in rats and was distributed to the fetus.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
The volume of distribution of olmesartan is approximately 17 L. Olmesartan is highly bound to plasma proteins (99%) and does not penetrate red blood cells. The protein binding is constant at plasma olmesartan concentrations well above the range achieved with recommended doses.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
Olmesartan medoxomil is rapidly and completely bioactivated by ester hydrolysis to olmesartan during absorption from the gastrointestinal tract. Benicar tablets and the suspension formulation prepared from Benicar tablets are bioequivalent. The absolute bioavailability of olmesartan is approximately 26%. After oral administration, the peak plasma concentration (Cmax ) of olmesartan is reached after 1 to 2 hours. Food does not affect the bioavailability of olmesartan.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
Total plasma clearance of olmesartan is 1.3 L/hr, with a renal clearance of 0.6 L/hr. Approximately 35% to 50% of the absorbed dose is recovered in urine while the remainder is eliminated in feces via the bile. ... Olmesartan shows linear pharmacokinetics following single oral doses of up to 320 mg and multiple oral doses of up to 80 mg. Steady-state levels of olmesartan are achieved within 3 to 5 days and no accumulation in plasma occurs with once-daily dosing.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
Olmesartan is distributed into milk in rats; it is not known whether the drug is distributed into milk in humans.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2027
Olmesartan medoxomil is rapidly and completely bioactivated by ester hydrolysis to olmesartan during absorption from the gastrointestinal tract. This rapid first-pass metabolism was confirmed by the lack of measurable amounts of olmesartan medoxomil in plasma or excreta. This first-pass metabolism is not driven by cytochrome enzymes and hence it is not expected to interact with other drugs via this mechanism. The pharmacologically active moiety does not appear to undergo further metabolism.
Following the rapid and complete conversion of olmesartan medoxomil to olmesartan during absorption, there is virtually no further metabolism of olmesartan.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
The mean plasma olmesartan half-life is reported to be from 10-15 hours after multiple oral administration.
Olmesartan appears to be eliminated in a biphasic manner with a terminal elimination half-life of approximately 13 hours.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b
Olmesartan belongs to the angiotensin II receptor blocker (ARB) family of drugs, which also includes [telmisartan], [candesartan], [losartan], [valsartan], and [irbesartan]. ARBs selectively bind to angiotensin receptor 1 (AT1) and prevent the protein angiotensin II from binding and exerting its hypertensive effects. As the principal pressor agent of the renin-angiotensin system, Angiotensin II causes vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation and renal reabsorption of sodium. Olmesartan blocks the vasoconstrictor effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in vascular smooth muscle. Its action is, therefore, independent of the pathways for angiotensin II synthesis. Overall, olmesartan's physiologic effects lead to reduced blood pressure, lower aldosterone levels, reduced cardiac activity, and increased excretion of sodium. Olmesartan also effects on the renin-angiotensin aldosterone system (RAAS) plays an important role in hemostasis and regulation of kidney, vascular, and cardiac functions. Pharmacological blockade of RAAS via AT1 receptor blockade inhibits negative regulatory feedback within RAAS, which is a contributing factor to the pathogenesis and progression of cardiovascular disease, heart failure, and renal disease. In particular, heart failure is associated with chronic activation of RAAS, leading to inappropriate fluid retention, vasoconstriction, and ultimately a further decline in left ventricular function. ARBs have been shown to have a protective effect on the heart by improving cardiac function, reducing afterload, increasing cardiac output and preventing ventricular hypertrophy and remodelling.
Angiotensin-converting enzyme 2 (ACE2) is highly expressed in the kidney and converts angiotensin (Ang) II to Ang-(1-7), a renoprotective peptide. Urinary ACE2 has been shown to be elevated in patients with chronic kidney disease. However, the effects of antihypertensive agents on urinary ACE2 remain unclear. METHODS: Of participants in the Tanno-Sobetsu cohort study in 2011 (n = 617), subjects on no medication (n = 101) and hypertensive patients treated with antihypertensive agents, including the calcium channel blockers amlodipine and long-acting nifedipine; the ACE inhibitor enalapril; and the Ang II receptor blockers losartan, candesartan, valsartan, telmisartan, and olmesartan, for more than 1 year (n = 100) were enrolled, and urinary ACE2 level was measured. Glucose and hemoglobin A1c were significantly higher in patients treated with enalapril, telmisartan or olmesartan than in the control subjects. Urinary albumin-to-creatinine ratio (UACR) was significantly higher in patients treated with enalapril than in the control subjects. Urinary ACE2 level was higher in the olmesartan-treated group, but not the other treatment groups, than in the control group. Urinary ACE2 level was positively correlated with systolic blood pressure (r = 0.211; P = 0.003), UACR (r = 0.367; P < 0.001), and estimated salt intake (r = 0.260; P < 0.001). Multivariable regression analysis after adjustment of age, sex, and the correlated indices showed that the use of olmesartan was an independent predictor of urinary ACE2 level. In contrast with other antihypertensive drugs, olmesartan may uniquely increase urinary ACE2 level, which could potentially offer additional renoprotective effects.
PMID:24842388 Furuhashi M et al; Am J Hypertens. 2014 May 18. pii: hpu086. (Epub ahead of print)
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation and renal reabsorption of sodium. Olmesartan blocks the vasoconstrictor effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT receptor in vascular smooth muscle. Its action is, therefore, independent of the pathways for angiotensin II synthesis. An AT receptor is found also in many tissues, but this receptor is not known to be associated with cardiovascular homeostasis. Olmesartan has more than a 12,500-fold greater affinity for the AT receptor than for the AT receptor. Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is a mechanism of many drugs used to treat hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because olmesartan medoxomil does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and circulating angiotensin II levels do not overcome the effect of olmesartan on blood pressure.
NIH; DailyMed. Current Medication Information for Benicar (Olmesartan Medoxomil) Tablet, Film-coated (Revised: September 2014). Available from, as of October 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33770d80-754f-11de-8dba-0002a5d5c51b