


1. Glipressin
2. Gly Gly Gly 8 Lys Vasopressin
3. Gly-gly-gly-8-lys-vasopressin
4. Glycylpressin
5. Glypressin
6. N-(alpha)-glycyl-glycyl-glycyl-8-lysine Vasopressin
7. Remestyp
8. Terlypressin
9. Tglvp
10. Tri-gly-8-lys- Vasopressin
11. Triglycyl Lysine Vasopressin
12. Triglycyl-(8-lysine)vasopressin
13. Triglycylvasopressin
14. Vasopressin, Tri-gly-8-lys-
1. 14636-12-5
2. Terlipressin Acetate
3. Glypressin
4. Glycylpressin
5. Terlipressine [inn-french]
6. Terlipressinum [inn-latin]
7. Terlipressina [inn-spanish]
8. N-(n-(n-glycylglycyl)glycyl)-8-l-lysinevasopressin
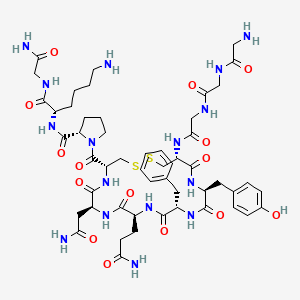
9. (2s)-1-[(4r,7s,10s,13s,16s,19r)-19-[[2-[[2-[(2-aminoacetyl)amino]acetyl]amino]acetyl]amino]-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[(2s)-6-amino-1-[(2-amino-2-oxoethyl)amino]-1-oxohexan-2-yl]pyrrolidine-2-carboxamide
10. Ncgc00185754-01
11. Terlipressina
12. Terlipressine
13. Terlipressinum
14. Lucassin
15. Einecs 238-680-8
16. Terlivaz
17. Variquel
18. Unii-7z5x49w53p
19. Terlipressin [usan:inn:ban]
20. Lucassin (tn)
21. Hs-2028
22. Terlipressin (usan/inn)
23. Dsstox_cid_28878
24. Dsstox_rid_83146
25. Dsstox_gsid_48952
26. Schembl22699
27. Chembl2135460
28. Dtxsid7048952
29. Gtpl11241
30. Chebi:135905
31. 7z5x49w53p
32. Amy25370
33. Ex-a3116
34. Glycyl-glycyl-glycyl-l-cysteinyl-l-tyrosyl-l-phenylalanyl-l-glutaminyl-l-asparagyl-l-cysteinyl-l-prolyl-l-lysyl-glycinamide (4->9)-disulfide
35. Tox21_113374
36. Akos015994637
37. Ccg-270662
38. Cs-5769
39. Db02638
40. Hy-12554
41. Cas-14636-12-5
42. 36t125
43. C72780
44. D06672
45. Q324147
46. J-008213
47. (2s)-6-amino-2-{[(2s)-1-{[(4r,7s,10s,13s,16s,19r)-19-{2-[2-(2-aminoacetamido)acetamido]acetamido}-13-benzyl-10-(2-carbamoylethyl)-7-(carbamoylmethyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl]carbonyl}pyrrolidin-2-yl]formamido}-n-(carbamoylmethyl)hexanamide
| Molecular Weight | 1227.4 g/mol |
|---|---|
| Molecular Formula | C52H74N16O15S2 |
| XLogP3 | -5.8 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 25 |
| Exact Mass | 1226.49609806 g/mol |
| Monoisotopic Mass | 1226.49609806 g/mol |
| Topological Polar Surface Area | 563 Ų |
| Heavy Atom Count | 85 |
| Formal Charge | 0 |
| Complexity | 2380 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Commonly used to stop bleeding of varices in the food pipe (oesophagus).
Terlipressin is a medicine similar to a naturally occurring hormone present in the body, known as antidiuretic hormone (ADH) or vasopressin. ADH has two main effects in the body. Firstly, it causes narrowing of blood vessels (vasoconstriction), thereby limiting blood flow to a particular area of the body. It also acts on receptors in the kidney to retain water in the body, which helps to prevent excessive loss of water in the urine.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
H01BA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
H - Systemic hormonal preparations, excl. sex hormones and insulins
H01 - Pituitary and hypothalamic hormones and analogues
H01B - Posterior pituitary lobe hormones
H01BA - Vasopressin and analogues
H01BA04 - Terlipressin
Terlipressin, an analogue of vasopressin, acts on three different receptors, vasopressin receptor V1a (which initiates vasoconstriction, liver gluconeogenesis, platelet aggregation and release of factor VIII), vasopressin receptor V1b (which mediates corticotrophin secretion from the pituitary) and vasopressin receptor V2 which controls free water reabsorption in the renal medullar. The binding of terlipressin to the V2 receptor activates adenylate cyclase which causes the release of aquaporin 2 channels into the cells lining the renal medullar duct. This allows water to be reabsorbed down an osmotic gradient so the urine is more concentrated.