

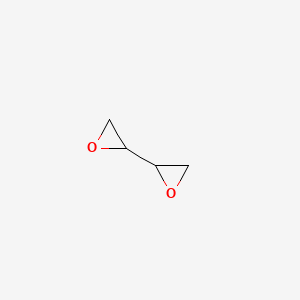

1. 1,2,3,4-diepoxybutane
2. 1,2-3,4-diepoxybutane
3. Butadiene Bisoxide
4. Butadiene Diepoxide
5. Butadiene Dioxide
6. Diepoxybutane
7. Diepoxybutane, Meso-
8. Erythritol Anhydride
9. Erythritol Anhydride, ((r*,r*)-(+-))-isomer
10. Erythritol Anhydride, (r*,s*)-isomer
11. Erythritol Anhydride, (r-(r*,r*))-isomer
12. Erythritol Anhydride, (s-(r*,r*))-isomer
13. Meso-diepoxybutane
1. Diepoxybutane
2. 1,3-butadiene Diepoxide
3. 1464-53-5
4. Butadiene Diepoxide
5. Butadiene Dioxide
6. Dioxybutadiene
7. Bioxirane
8. Butane Diepoxide
9. 1,2:3,4-diepoxybutane
10. Bioxiran
11. 2-(oxiran-2-yl)oxirane
12. Butadiendioxyd
13. 1,2:3,4-butadiene Diepoxide
14. Butane, 1,2:3,4-diepoxy-
15. 1,2,3,4-diepoxybutane
16. Rcra Waste Number U085
17. 1,1'-bi(ethylene Oxide)
18. 1,1'-bi[ethylene Oxide]
19. Cb 1181
20. Nsc 629
21. Threitol, 1,2:3,4-dianhydro-
22. R 181
23. Ent-26592
24. M 8838
25. D,l-diepoxybutane
26. Chebi:23704
27. D,l-1,2:3,4-diepoxybutane
28. 298-18-0
29. Unii-60ob65ynab
30. Butadiendioxyd [german]
31. Ccris 234
32. Butane,2:3,4-diepoxy-
33. Hsdb 4046
34. Einecs 215-979-1
35. Rcra Waste No. U085
36. Wln: T3otj B- Bt3otj
37. Brn 0079833
38. 1,2,3,4-diepoxybutane Dl
39. Ai3-26592
40. 1,4-diepoxybutane
41. 2,4-diepoxybutane
42. 2,2'-bioxirane, (r*,r*)-(.+/-.)-
43. 1,3,4-diepoxybutane
44. 60ob65ynab
45. 1,2:3,4-dianhydrothreitol
46. Threitol,2:3,4-dianhydro-
47. 5-19-01-00185 (beilstein Handbook Reference)
48. Nsc629
49. Chembl1964283
50. Dtxsid0041307
51. 1,3-butadiene Diepoxide, 97%
52. Zfivkaoqexoyfy-uhfffaoysa-
53. Nsc-629
54. 5-(dimetnmet)furfuryl Alcohol Hcl
55. (a+/-)-1,2;3,4-diepoxy-butane
56. Akos017342854
57. 2,2 Inverted Exclamation Marka-bioxirane
58. Nci60_009412
59. 1,3-butadiene Diepoxide, Analytical Standard
60. B0234
61. Cs-0377906
62. D3410
63. Ft-0606590
64. Butane, 1,2:3,4-diepoxy-, (.+/-.)-
65. J-503880
66. J-640017
67. J-800012
68. Q5274970
69. Erythrithol Anhydride (old Name); 1,3-butadiene Diepoxide
| Molecular Weight | 86.09 g/mol |
|---|---|
| Molecular Formula | C4H6O2 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 86.036779430 g/mol |
| Monoisotopic Mass | 86.036779430 g/mol |
| Topological Polar Surface Area | 25.1 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 61.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Carcinogens
Substances that increase the risk of NEOPLASMS in humans or animals. Both genotoxic chemicals, which affect DNA directly, and nongenotoxic chemicals, which induce neoplasms by other mechanism, are included. (See all compounds classified as Carcinogens.)
Cross-Linking Reagents
Reagents with two reactive groups, usually at opposite ends of the molecule, that are capable of reacting with and thereby forming bridges between side chains of amino acids in proteins; the locations of naturally reactive areas within proteins can thereby be identified; may also be used for other macromolecules, like glycoproteins, nucleic acids, or other. (See all compounds classified as Cross-Linking Reagents.)
Mutagens
Chemical agents that increase the rate of genetic mutation by interfering with the function of nucleic acids. A clastogen is a specific mutagen that causes breaks in chromosomes. (See all compounds classified as Mutagens.)
Experiments were carried out to study the uptake, retention, routes of elimination, and identification of butadiene metabolites in rats and mice. Male Sprague-Dawley rats and male B6C3F1 mice were administered (14)C labeled butadiene by inhalation at 0.14 to 13000 microg/l air. The percentage of (14)C absorbed and retained at 6 hours after exposure was 1.5 to 17 in rats and 4 to 20 in mice. Major routes of elimination were exhalation and urine discharge, accounting for 75 to 85% of the total (14)C. About 12% of (14)C remained in the carcass 65 hours after the exposure. At the 13 microg/l dose, all (14)C was accounted for as metabolites. At the lowest butadiene concentrations (14)C in urine and exhaled air represented 40 and 20% of the total (14)C eliminated. At the highest butadiene concentration (14)C in urine decreased to 8 % of total (14)C eliminated or retained in carcass. More than 90% of (14)C in blood samples consisted of butadiene metabolites, of which 60 to 80 % was nonvolatile material. The amount of metabolites increased with the concentration of inhaled butadiene, but the increase was not proportional to the uptake. At 130 and 1800 microg/l butadiene mice had about twofold higher amount of 1,2-epoxy-3-butene than rats. The quantities of butadiene-diepoxide in rats subjected to the same exposure levels of butadiene were 0.1 and 1 nmol/ml blood after 6 hours. /Butadiene/
PMID:3726881 Bond JA et al; Toxicol Appl Pharmacol 84 (3): 617-627 (1986)
Experiments were carried out to study the uptake, retention, routes of elimination, and identification of butadiene metabolites in rats and mice. Male Sprague-Dawley rats and male B6C3F1 mice were administered (14)C labeled butadiene by inhalation at 0.14 to 13000 microg/l air. The percentage of (14)C absorbed and retained at 6 hours after exposure was 1.5 to 17 in rats and 4 to 20 in mice. Major routes of elimination were exhalation and urine discharge, accounting for 75 to 85% of the total (14)C. About 12% of (14)C remained in the carcass 65 hours after the exposure. At the 13 microg/l dose, all (14)C was accounted for as metabolites. At the lowest butadiene concentrations (14)C in urine and exhaled air represented 40 and 20% of the total (14)C eliminated. At the highest butadiene concentration (14)C in urine decreased to 8 % of total (14)C eliminated or retained in carcass. More than 90% of (14)C in blood samples consisted of butadiene metabolites, of which 60 to 80 % was nonvolatile material. The amount of metabolites increased with the concentration of inhaled butadiene, but the increase was not proportional to the uptake. At 130 and 1800 microg/l butadiene mice had about twofold higher amount of 1,2-epoxy-3-butene than rats. The quantities of butadiene-diepoxide in rats subjected to the same exposure levels of butadiene were 0.1 and 1 nmol/ml blood after 6 hours. /Butadiene/
PMID:3726881 Bond JA et al; Toxicol Appl Pharmacol 84 (3): 617-627 (1986)
Diepoxybutane (DEB) is a known human metabolite of butadiene_monoxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560