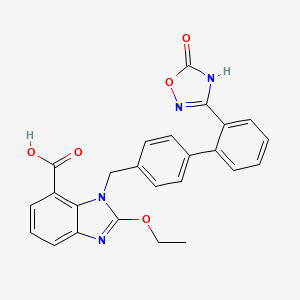

1. 2-ethoxy-1-((2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-biphenyl-4-yl)methyl)-1h-benzimidazole-7-carboxylic Acid
2. Tak-536
1. 147403-03-0
2. Tak-536
3. Tak 536
4. Azilsartan (tak-536)
5. Chembl57242
6. F9nux55p23
7. Tak536
8. 2-ethoxy-3-[[4-[2-(5-oxo-2h-1,2,4-oxadiazol-3-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic Acid
9. Chebi:68850
10. 2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1h-benzimidazole-7-carboxylic Acid
11. 2-ethoxy-1-((2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl)methyl)-1h-benzo[d]imidazole-7-carboxylic Acid
12. 1h-benzimidazole-7-carboxylic Acid, 1-((2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol- 3-yl)(1,1'-biphenyl)-4-yl)methyl)-2-ethoxy-
13. 2-ethoxy-3-[[4-[2-(5-oxo-4h-1,2,4-oxadiazol-3-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic Acid
14. 2-ethoxy-3-[2'-(5-oxo-4,5-dihydro-[1,2,4]oxadiazol-3-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid
15. Azilsartan [usan]
16. Azilsartan [usan:inn]
17. Unii-f9nux55p23
18. Hsdb 8208
19. Azilsartan- Bio-x
20. Azilsartan Free Acid
21. Azilva (tn)
22. Azilsartan [mi]
23. Azilsartan [inn]
24. Azilsartan [jan]
25. Azilsartan [vandf]
26. Azilsartan [mart.]
27. Azilsartan [who-dd]
28. 2-ethoxy-1-[[2'-(4,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl]benzimidazole-7-carboxylic Acid
29. Azilsartan (jan/usan/inn)
30. Schembl167538
31. Gtpl6901
32. Azilsartan, >=98% (hplc)
33. Amy4360
34. Dtxsid70163712
35. Hms3651j16
36. Hms3747i09
37. Zinc598390
38. Bcp03826
39. Wdc59945
40. Bdbm50055441
41. Cx1016
42. Mfcd20278186
43. S3046
44. Akos007930882
45. Akos024458220
46. Am84439
47. Bcp9000002
48. Ccg-269296
49. Cs-1396
50. Ks-5381
51. Sb20804
52. Ncgc00386206-03
53. Ncgc00386206-04
54. 1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl]-4-yl]methyl]-2-ethoxy-1h-benzimidazole-7-carboxylic Acid
55. 2-ethoxy-1-((2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-biphenyl-4-yl)methyl)-1h-benzimidazole-7-carboxylic Acid
56. Ac-25912
57. Ba164233
58. Hy-14914
59. Bcp0726000135
60. Db-063707
61. Ft-0662457
62. Ft-0662458
63. Sw219493-1
64. D08864
65. Ab01566867_01
66. 403a030
67. L001451
68. Q27074832
69. 1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl) [1,1'-biphenyl]-4-yl]methyl]-2-ethoxy-1h-benzimidazole-7-carboxylic Acid
70. 2-ethoxy-1-((2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl)methyl)-1h-benzimidazole-7-carboxylic Acid
71. 2-ethoxy-1-((2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl)methyl)- 1h-benzimidazole-7-carboxylic Acid
72. 2-ethoxy-1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl]benzimidazole-7-carboxylic Acid
73. 2-ethoxy-1-[[2'-(2,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)biphenyl4-yl]methyl]benzimidazole-7-carboxylic Acid
74. 2-ethoxy-3-[2''-(5-oxo-4,5-dihydro-[1,2,4]oxadiazol-3-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid
75. 2-ethoxy-3-[2'-(5-oxo-2,5-dihydro-[1,2,4]oxadiazol-3-yl)-biphenyl-4-ylmethyl]-3h-benzoimidazole-4-carboxylic Acid
| Molecular Weight | 456.4 g/mol |
|---|---|
| Molecular Formula | C25H20N4O5 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 456.14336975 g/mol |
| Monoisotopic Mass | 456.14336975 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 783 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Edarbi is an angiotensin II receptor blocker (ARB) indicated for the treatment of hypertension to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including the class to which this drug principally belongs. /Included in US product label/
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
Edarbi may be used alone or in combination with other antihypertensive agents.
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
Both angiotensin II receptor antagonists /eg, azilsartan/ and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2056
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Edarbi as soon as possibl. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
Drugs that act directly on the renin-angiotensin system (e.g., ACE inhibitors, angiotensin II receptor antagonists) reduce fetal renal function and increase fetal and neonatal morbidity and mortality when used in pregnancy during the second and third trimesters. ACE inhibitors also may increase the risk of major congenital malformations when administered during the first trimester of pregnancy. Azilsartan should be discontinued as soon as possible when pregnancy is detected, unless continued use is considered life-saving. Nearly all women can be transferred successfully to alternative therapy for the remainder of their pregnancy.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2057
Use of drugs that affect the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Edarbi as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
Because symptomatic hypotension may occur in patients with an activated renin-angiotensin system (e.g., patients with volume or salt depletion secondary to high doses of diuretics), azilsartan should be initiated in such patients after volume or salt depletion is corrected, or a lower initial dose of the drug should be used. If hypotension occurs in patients receiving azilsartan medoxomil, the patient should be placed in the supine position and, if necessary, an IV infusion of 0.9% sodium chloride injection should be administered. Transient hypotension is not a contraindication to additional doses of azilsartan, and therapy with the drug can be cautiously reinstated after blood pressure has been stabilized (e.g., with volume expansion).
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2057
For more Drug Warnings (Complete) data for Azilsartan (14 total), please visit the HSDB record page.
In rats, minimal azilsartan-associated radioactivity crossed the blood-brain barrier. Azilsartan passed across the placental barrier in pregnant rats and was distributed to the fetus.
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
The volume of distribution of azilsartan is approximately 16 L. Azilsartan is highly bound to human plasma proteins (>99%), mainly serum albumin. Protein binding is constant at azilsartan plasma concentrations well above the range achieved with recommended doses.
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
Following an oral dose of C-labeled azilsartan medoxomil, approximately 55% of radioactivity was recovered in feces and approximately 42% in urine, with 15% of the dose excreted in urine as azilsartan. The elimination half-life of azilsartan is approximately 11 hours and renal clearance is approximately 2.3 mL/min. Steady-state levels of azilsartan are achieved within five days, and no accumulation in plasma occurs with repeated once-daily dosing.
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
Azilsartan medoxomil is hydrolyzed to azilsartan, the active metabolite, in the gastrointestinal tract during absorption. Azilsartan medoxomil is not detected in plasma after oral administration. Dose proportionality in exposure was established for azilsartan in the azilsartan medoxomil dose range of 20 mg to 320 mg after single or multiple dosing. The estimated absolute bioavailability of azilsartan following administration of azilsartan medoxomil is approximately 60%. After oral administration of azilsartan medoxomil, peak plasma concentrations (Cmax) of azilsartan are reached within 1.5 to 3 hours. Food does not affect the bioavailability of azilsartan.
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
For more Absorption, Distribution and Excretion (Complete) data for Azilsartan (8 total), please visit the HSDB record page.
Azilsartan medoxomil is rapidly hydrolysed to the active moiety azilsartan by esterases in the gastrointestinal tract and/or during drug absorption. Based on vitro studies, the enzymes involved in the hydrolysis of azilsartan medoxomil to azilsartan in human plasma, and in human liver and small intestinal cytosol seem to be similar to those involved in the hydrolysis of olmesartan medoxomil. Currently, no drug interactions are listed for the hydrolysis of azilsartan medoxomil. The enzyme carboxymethylenebutenolidase is a recently discovered hydrolysis mechanism for azilsartan medoxomi in the intestine and liver, but no interactions with other drugs have been reported for this enzyme in the Metabolism and Transport Drug Interaction Database (DIDB). Also no interactions have been reported for human serum albumin or arylesterases. Since there are multiple esterase pathways involved in the conversion of azilsartan medoxomil to azilsartan, the potential for interactions via this pathway is considered to be minimal. The metabolites M-I and M-II were formed by decarboxylation and dealkylation of azilsartan, respectively, and are pharmacologically inactive. CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 are all capable of metabolising azilsartan. However, CYP2C9 showed the highest activity in metabolising azilsartan to M-II and CYP2C8 in metabolising azilsartan to M-I.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Edarbi (Azilsartan Medoxomil) p.16 (2011). Available from, as of July 15, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002293/WC500119206.pdf
Azilsartan is metabolized to two primary metabolites. The major metabolite in plasma is formed by O-dealkylation, referred to as metabolite M-II, and the minor metabolite is formed by decarboxylation, referred to as metabolite M-I. Systemic exposures to the major and minor metabolites in humans were approximately 50% and less than 1% of azilsartan, respectively. M-I and M-II do not contribute to the pharmacologic activity of Edarbi. The major enzyme responsible for azilsartan metabolism is CYP2C9.
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
The half-life of azilsartan in plasma was between 4 and 6 hr in rats and dogs and approximately 12 hr in humans.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Edarbi (Azilsartan Medoxomil) p.15 (2011). Available from, as of July 15, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002293/WC500119206.pdf
The elimination half-life of azilsartan is approximately 11 hours ... .
NIH; DailyMed. Current Medication Information for Edarbi (Azilsartan Kamedoxomil) Tablet (Revised: October 2012). Available from, as of July 10, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b16bfc-38c1-4133-bd7d-13258d54edec
Azilsartan medoxomil is a newly approved angiotensin receptor blocker (ARB) reported to lower 24 hr blood pressure more effectively than maximally recommended doses of older ARBs. Although azilsartan is considered to be an unusually potent angiotensin II type 1 (AT1) receptor antagonist, little is known about the potential pleiotropic effects of this molecule. /The purpose of this study was to investigate/ pleiotropic features of azilsartan in cell-based assay systems independent of its effects on blood pressure. In cultured 3T3-L1 preadipocytes, azilsartan enhanced adipogenesis and exerted greater effects than valsartan on expression of genes encoding peroxisome proliferator-activated receptor-a (PPARa), PPARd, leptin, adipsin, and adiponectin. The effects of azilsartan on adipocyte differentiation and gene expression were observed at concentrations of azilsartan that did not classically stimulate PPAR activity in cell-based transactivation assays. Azilsartan also potently inhibited vascular cell proliferation in the absence of exogenously supplemented angiotensin II. In aortic endothelial cells, azilsartan inhibited cell proliferation at concentrations as low as 1 umol/L, whereas valsartan showed little or no antiproliferative effects at concentrations below 10 umol/L. Antiproliferative effects of azilsartan were also observed in cells lacking AT1 receptors. In addition, azilsartan, but not valsartan, blocked angiotensin II-induced activation of mitogen-activated protein kinase in vascular smooth muscle cells 4-8 hr after washout of drug from the incubation media. These findings suggest that azilsartan can function as a pleiotropic ARB with potentially beneficial effects on cellular mechanisms of cardiometabolic disease through actions that could involve more than just blockade of AT1 receptors and/or reduction in blood pressure.
PMID:21986624 Kajiya T et al; J Hypertens 29 (12): 2476-83 (2011)
Angiotensin receptor (type 1) blockers (ARBs) can reduce both hypertension and insulin resistance induced by local and systemic activation of the renin-angiotensin-aldosterone system. The effectiveness of azilsartan medoxomil (AZIL-M), a novel imidazole-based ARB, to facilitate metabolic improvements in conditions of angiotensin II (Ang II)-associated insulin resistance is currently unknown. The aim of this study was to determine the impact of chronic AZIL-M treatment on glucose transport activity and key insulin signaling elements in red skeletal muscle of Ang II-treated rats. Male Sprague-Dawley rats were treated for 8 weeks with or without Ang II (200 ng/kg/min) combined with either vehicle or AZIL-M (1 mg/kg/day). Ang II induced significant (p < 0.05) increases in blood pressure, which were completely prevented by AZIL-M. Furthermore, Ang II reduced insulin-mediated glucose transport activity in incubated soleus muscle, and AZIL-M co-treatment increased this parameter. Moreover, AZIL-M treatment of Ang II-infused animals increased the absolute phosphorylation of insulin signaling molecules, including Akt [both Ser473 (81%) and Thr308 (23%)] and AS160 Thr642 (42%), in red gastrocnemius muscle frozen in situ. Absolute AMPKalpha (Thr172) phosphorylation increased (98%) by AZIL-M treatment, and relative Thr389 phosphorylation of p70 S6K1, a negative regulator of insulin signaling, decreased (51%) with AZIL-M treatment. These results indicate that ARB AZIL-M improves the in vitro insulin action on glucose transport in red soleus muscle and the functionality of the Akt/AS160 axis in red gastrocnemius muscle in situ in Ang II-induced insulin-resistant rats, with the latter modification possibly associated with enhanced AMPKalpha and suppressed p70 S6K1 activation.
PMID:23922555 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3721134 Lastra G et al; Cardiorenal Med 3 (2): 154-164 (2013)