
1. Decaris
2. Dekaris
3. Hydrochloride, Levamisole
4. L-tetramisole
5. Levamisole
6. Levotetramisole
7. Solaskil
1. 16595-80-5
2. Levamisole Hcl
3. Ergamisol
4. (s)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole Hydrochloride
5. Ascaridil
6. Tramisol
7. (-)-tetramisole Hydrochloride
8. Tramisole
9. Decaris
10. Niratic Hydrochloride
11. Stimamizol Hydrochloride
12. (-)-tetramisole
13. Nsc-177023
14. Niratic-puron Hydrochloride
15. Spartakon L
16. L-narpenol
17. Levamisole (hydrochloride)
18. R 12,564
19. Spartakon
20. R 12564
21. R-12,564
22. Chebi:6433
23. Tetramisole Hydrochloride, (s)-
24. (-)-2,3,5,6-tetrahydro-6-phenylimidazo(2,1-b)thiazole Monohydrochloride
25. Imidazo(2,1-b)thiazole, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride, (s)-
26. Dl9055k809
27. Dekaris
28. Levacide
29. Levadin
30. Levasole
31. Nemicide
32. Solaskil
33. Meglum
34. Nilverm Forte
35. Worm-chek
36. (6s)-6-phenyl-2h,3h,5h,6h-imidazo[2,1-b][1,3]thiazole Hydrochloride
37. Ripercol-l
38. Citarin L
39. 16595-80-5 (hcl)
40. Dsstox_cid_27518
41. Dsstox_rid_82391
42. Dsstox_gsid_47518
43. R 12654
44. R-12564
45. 74191-78-9
46. Levomysol Hydrochloride
47. (6s)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b][1,3]thiazole;hydrochloride
48. (7s)-7-phenyl-4-thia-1,6-diazabicyclo[3.3.0]oct-5-ene Hydrochloride
49. Kw 2le-t
50. Smr001230807
51. Kw-2-le-t
52. Nsc177023
53. Levamisole Hydrochloride [usan]
54. Levamisol Hydrochloride
55. Sr-01000075969
56. Einecs 240-654-6
57. L-tetramisole Hydrochloride
58. (-)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole Hydrochloride
59. Unii-dl9055k809
60. Lev Hydrochloride
61. Ergamisol (tn)
62. Ncgc00094446-03
63. Prestwick_294
64. Levamisole Hydrochloride [usan:usp]
65. Mfcd00012675
66. Levamisoli Hydrochloridum
67. Levamisole Hcl(ergamisol)
68. L-(-)-2,3,5,6-tetrahydro-6-phenyl-imidazo(2,1-b)thiazole Hydrochloride
69. Ergamisol (tn) (janssen)
70. Cas-16595-80-5
71. Chembl1770
72. Schembl19226
73. Bmk1-h6
74. Levamisole Hydrochloride,(s)
75. Imidazo[2, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride
76. Mls002153440
77. Mls002222198
78. Mls002695941
79. Spectrum1503245
80. Levamisole Hydrochloride (usp)
81. ((-)-tetramisole Hydrochloride
82. Dtxsid7047518
83. Hms1568f05
84. Hms1922m11
85. Bcp13755
86. Levamisole Hydrochloride (ergamisol)
87. Tox21_111282
88. Tox21_500690
89. Ccg-40301
90. Levamisole Hydrochloride [mi]
91. S1939
92. Levamisole Hydrochloride [jan]
93. (s)-(-)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazole Hydrochloride
94. Akos015951350
95. L(-)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole Hydrochloride
96. Tox21_111282_1
97. Kw-2299
98. Lp00690
99. Imidazo(2,1-b)thiazole, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride, (-)-
100. Imidazo(2,1-b)thiazole, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride, L-(-)-
101. Levamisole Hydrochloride [mart.]
102. Levamisole Hydrochloride [usp-rs]
103. Levamisole Hydrochloride [who-dd]
104. Levamisole Hydrochloride [who-ip]
105. Ncgc00094046-01
106. Ncgc00261375-01
107. Ncgc00263461-01
108. Ac-23973
109. As-13370
110. Bl166245
111. Hy-13666
112. Eu-0100690
113. L0231
114. Levamisole Hydrochloride [green Book]
115. T1215
116. (-)-tetramisole Hydrochloride, >=99% (gc)
117. Levamisole Hydrochloride [orange Book]
118. C07906
119. D00750
120. D78137
121. L 9756
122. Levamisole Hydrochloride [ep Monograph]
123. Levamisole Hydrochloride [usp Monograph]
124. Levamisoli Hydrochloridum [who-ip Latin]
125. J-010244
126. J-521642
127. Levamisole Hydrochloride 100 Microg/ml In Methanol
128. Sr-01000075969-1
129. Sr-01000075969-6
130. Levamisol Hydrochloride 100 Microg/ml In Acetonitrile
131. Q27107205
132. Z1550648764
133. Levamisol Hydrochloride, Vetranal(tm), Analytical Standard
134. Levamisole Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
135. Imidazo[2, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride, (-)-
136. Imidazo[2, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride, (s)-
137. (-)-2,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole Monohydrochloride
138. (s)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiazolehydrochloride
139. Imidazo[2, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride, L-(-)-
140. Levamisole Hydrochloride, European Pharmacopoeia (ep) Reference Standard
141. Imidazo(2,1-b)thiazole, 2,3,5,6-tetrahydro-6-phenyl-, Monohydrochloride, (6s)-
142. Levamisole Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
143. Levamisole Hydrochloride, United States Pharmacopeia (usp) Reference Standard
144. Levamisole Hydrochloride For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 240.75 g/mol |
|---|---|
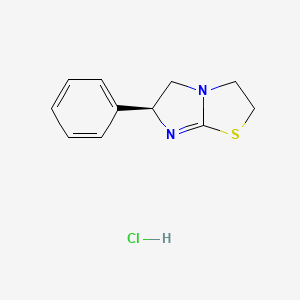
| Molecular Formula | C11H13ClN2S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 240.0487973 g/mol |
| Monoisotopic Mass | 240.0487973 g/mol |
| Topological Polar Surface Area | 40.9 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Treatment of glomerulonephritis and nephrotic syndrome
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
Antinematodal Agents
Substances used in the treatment or control of nematode infestations. They are used also in veterinary practice. (See all compounds classified as Antinematodal Agents.)
Antirheumatic Agents
Drugs that are used to treat RHEUMATOID ARTHRITIS. (See all compounds classified as Antirheumatic Agents.)