


1. 4-phenylbutyrate
2. 4-phenylbutyric Acid, Calcium Salt
3. 4-phenylbutyric Acid, Sodium Salt
4. Ammonaps
5. Buphenyl
6. Sodium 4-phenylbutanoate
7. Sodium 4-phenylbutyrate
8. Sodium Phenylbutyrate
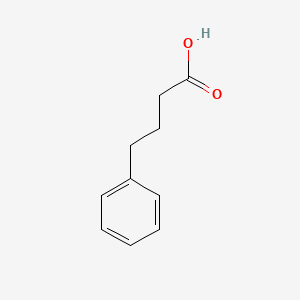
1. 4-phenylbutanoic Acid
2. 1821-12-1
3. Benzenebutanoic Acid
4. Benzenebutyric Acid
5. Phenylbutyrate
6. Phenylbutyric Acid
7. Gamma-phenylbutyric Acid
8. Omega-phenylbutanoic Acid
9. Butyric Acid, 4-phenyl-
10. 4-phenyl-butanoic Acid
11. 4-phenyl-n-butyric Acid
12. 1-phenylbutyric Acid
13. Gamma-phenyl-butyric Acid
14. 4-phenyl-butyric Acid
15. Omega-phenylbutyric Acid
16. Chebi:41500
17. Phenylbutanoic Acid
18. .gamma.-phenylbutyric Acid
19. Gamma-phenyl-n-butyric Acid
20. Mfcd00004403
21. .omega.-phenylbutanoic Acid
22. 7wy7ybi87e
23. Mls000069408
24. Pba
25. 4-phenylbutyric Acid (4-pba)
26. Nsc-295
27. Smr000059104
28. 4-phenyl Butyric Acid
29. Nsc 295
30. Gamma-phenylbutanoic Acid
31. Ncgc00018113-03
32. Einecs 217-341-8
33. Hdinhib_000004
34. Unii-7wy7ybi87e
35. Benzenebutyrate
36. Benzenebutanoate
37. Ai3-12065
38. G-phenylbutyrate
39. G-phenylbutanoate
40. W-phenylbutanoate
41. G-phenyl-butyrate
42. 4-phenyl-butyrate
43. 2-methyl-1-phenyl-propan-2-amine
44. Gamma-phenylbutyrate
45. G-phenylbutyric Acid
46. Gamma-phenylbutanoate
47. Omega-phenylbutanoate
48. 4-phenyl-n-butyrate
49. G-phenylbutanoic Acid
50. Gamma-phenyl-butyrate
51. W-phenylbutanoic Acid
52. 4-phenylbutyric Acid Sodium 100 Microg/ml In Acetonitrile:water
53. G-phenyl-butyric Acid
54. Spectrum_001331
55. 2ay7
56. 3tz2
57. Opera_id_387
58. Specplus_000814
59. Spectrum2_001798
60. Spectrum3_000782
61. Spectrum4_000092
62. Spectrum5_001003
63. 4-pba;benzenebutyric Acid
64. Bmse000701
65. Epitope Id:167707
66. Ec 217-341-8
67. Schembl1716
68. Chembl1469
69. 3-phenylpropylcarboxylic Acid
70. 4-phenolsulfonic Acid Sodium
71. 4-phenylbutyric Acid, 99%
72. Bspbio_002484
73. Kbiogr_000384
74. Kbioss_001811
75. Mls001076482
76. Divk1c_006910
77. Spbio_001755
78. .gamma.-phenyl-n-butyric Acid
79. Gtpl8480
80. Nsc295
81. Dtxsid2037631
82. Kbio1_001854
83. Kbio2_001811
84. Kbio2_004379
85. Kbio2_006947
86. Kbio3_001704
87. Obkxeaxtfzpchs-uhfffaoysa-
88. Zinc56568
89. Hms2234g14
90. Hms3259m07
91. Bcp10715
92. Cs-d1686
93. Hy-a0281
94. Str05306
95. Bdbm50480960
96. Ccg-39733
97. Cx1106
98. S3592
99. Stl164372
100. Akos000154540
101. Ac-3254
102. Am84635
103. Db06819
104. Nc00469
105. Ps-4322
106. Ncgc00018113-01
107. Ncgc00018113-02
108. Ncgc00018113-04
109. Ncgc00018113-18
110. Nci60_002455
111. Nci60_020145
112. Sy004771
113. Ft-0619401
114. Ft-0673736
115. P0643
116. En300-35719
117. C21793
118. A812651
119. Q-200507
120. Brd-k67102207-001-02-6
121. Brd-k67102207-236-01-0
122. Q27088364
123. F2190-0002
124. 1262970-43-3
| Molecular Weight | 164.20 g/mol |
|---|---|
| Molecular Formula | C10H12O2 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 164.083729621 g/mol |
| Monoisotopic Mass | 164.083729621 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 137 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adjunctive therapy for the management of chronic urea cycle disorders due to deficiencies in carbamylphosphate (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase. it is indicated in all neonatal- onset efficiency presenting within the first 28 days of life. Also indicated in patients with late-onset, presenting after the first month of life with a history of hyperammonemic encephalopathy.
FDA Label
Decreases elevated plasma ammonia glutamine levels
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Absorption
Under fasting condition the Cmax of a single orally ingested 5g tablet and 5g powder after 1 hour are respectively 218mcg/ml and 195mcg/ml. The effect of food on phenylbutyrate absorption is still unknown.
Route of Elimination
The major route of elimination is the kidneys as phenylacetylglutamine.
Volume of Distribution
The volume of distribution of phenylbutyrate is 0.2 l/kg.
Clearance
Within 24 hours 80-100% of the administered dose in eliminated in the urine as pheylacetylglutamine.
The overall disposition of sodium phenylbutyrate and its metabolites has not been characterized fully. However, the drug is known to be metabolized to phenylacetate and subsequently to phenylacetylglutamine. Metabolism of phenylbutyrate occurs mainly in liver and kidney.
For sodium phenylbutyrate the half life is 0.77 hours. For phenylacetate the half life is 1.15 hours.
Sodium phenylbutyrate is a pro-drug that is metabolized to the active compound phenylacetate. Phenylacetate conjuages with glutamine via acetylation reaction to form the product phenylacetylglutamine, which is excreted by the kidneys. This provides an alternative mechanism for waste nitrogen excretion.