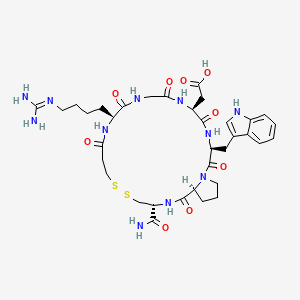

1. 7h-pyrrolo(2,1-g)(1,2,5,8,11,14,17,20)dithiahexaazacyclotricosine-17-acetic Acid, 3-(aminocarbonyl)-11-(4-((aminoiminomethyl)amino)butyl)docosahydro-20-(1h-indol-3-ylmethyl)-1,9,12,15,18,21-hexaoxo-, (3r,11s,17s,20s,25as)-
2. Epifibatide
3. Epifibratide
4. Eptifibatide
5. Integrelin
1. Eptifibatide
2. Integrelin
3. 188627-80-7
4. Chembl1174
5. Chebi:291902
6. 148031-34-9
7. Eptifibatide Acetate
8. Na8320j834
9. 2-[(3s,6s,12s,20r,23s)-20-carbamoyl-12-[4-(diaminomethylideneamino)butyl]-3-(1h-indol-3-ylmethyl)-2,5,8,11,14,22-hexaoxo-17,18-dithia-1,4,7,10,13,21-hexazabicyclo[21.3.0]hexacosan-6-yl]acetic Acid
10. Ncgc00167505-01
11. Intrifiban
12. C68-22
13. Dsstox_cid_26673
14. Dsstox_rid_81810
15. Dsstox_gsid_46673
16. [(3r,11s,17s,20s,25as)-11-(4-carbamimidamidobutyl)-3-carbamoyl-20-(1h-indol-3-ylmethyl)-1,9,12,15,18,21-hexaoxodocosahydro-7h-pyrrolo[2,1-g][1,2,5,8,11,14,17,20]dithiahexaazacyclotricosin-17-yl]acetic Acid
17. 157630-07-4
18. N(6)-amidino-n(2)-(3-mercaptopropionyl)-l-lysylglycyl-l-alpha-aspartyl-l-tryptophyl-l-prolyl-l-cysteinamide, Cyclic(1-6)-disulfide
19. N(sup 6)-amidino-n(sup 2)-(3-mercaptopropionyl)-l-lysylglycyl-l-alpha-aspartyl-l-tryptophyl-l-prolyl-l-cysteinamide, Cyclic(1-6)-disulfide
20. S(1),s(6)-cyclo[n(6)-carbamimidoyl-n(2)-(3-sulfanylpropanoyl)-l-lysylglycyl-l-alpha-aspartyl-l-tryptophyl-l-prolyl-l-cysteinamide]
21. 2-((3r,11s,17s,20s,25as)-20-((1h-indol-3-yl)methyl)-3-carbamoyl-11-(4-guanidinobutyl)-1,9,12,15,18,21-hexaoxodocosahydro-1h-pyrrolo[2,1-g][1,2,5,8,11,14,17,20]dithiahexaazacyclotricosin-17-yl)acetic Acid
22. L-cysteinamide, N6-(aminoiminomethyl)-n2-(3-mercapto-1-oxopropyl)-l-lysylglycyl-l-alpha-aspartyl-l-tryptophyl-l-prolyl-, Cyclic (1-->6)-disulfide
23. Cas-188627-80-7
24. Eptifibatide [inn]
25. Eptifibatide [inn:ban]
26. Sch-60936
27. Unii-na8320j834
28. Hs-2011
29. 1248559-53-6
30. Eptifibatide [mi]
31. Eptifibatide [usan]
32. Eptifibatide [vandf]
33. Schembl15781
34. Eptifibatide [mart.]
35. Eptifibatide [who-dd]
36. Eptifibatide Accord (generic)
37. Gtpl6585
38. Eptifibatide [ema Epar]
39. Dtxsid7046673
40. Hsdb 8313
41. Eptifibatide [orange Book]
42. Ex-a3111
43. Hy-b0686
44. Mpr-har-gly-asp-trp-pro-cys-nh2
45. Tox21_112503
46. Bdbm50092098
47. Zinc85536935
48. Akos015895205
49. Akos025147403
50. Tox21_112503_1
51. Ccg-270517
52. Ncgc00167505-03
53. Ncgc00263524-01
54. L-cysteinamide, N6-(aminoiminomethyl)-n2-(3-mercapto-1-oxopropyl)-l-lysylglycyl-l-alpha-aspartyl-l-tryptophy-l-prolyl-, Cyclic (1-6)-disulfide
55. 31e349
56. C73610
57. Q2295855
58. Z2241982440
59. [22-carbamoyl-14-(4-guanidino-butyl)-5-(1h-indol-3-ylmethyl)-4,7,10,13,16,24-hexaoxo-docosahydro-19,20-dithia-3a,6,9,12,15,23-hexaaza-cyclopentacyclotricosen-8-yl]-acetic Acid (c68-22, Eptifibatide)
60. 2-((3r,11s,17s,20s,25as)-20-((1h-indol-3-yl)methyl)-3-carbamoyl-11-(4-guanidinobutyl)-1,9,12,15,18,21-hexaoxodocosahydro-7h-pyrrolo[2,1-g][1,2]dithia[5,8,11,14,17,20]hexaazacyclotricosin-17-yl)acetic Acid
61. 2-[(3r,11s,17s,20s,25as)-11-(4-carbamimidamidobutyl)-3-carbamoyl-20-(indol-3-ylmethyl)-1,9,12,15,18,21-hexaoxo-docosahydro-1h-pyrrolo[2,1-g]1,2-dithia-5,8,11,14,17,20-hexaazacyclotricosan-17-yl]acetic Acid
62. 2-[(3r,11s,17s,20s,25as)-3-carbamoyl-11-{4-[(diaminomethylidene)amino]butyl}-20-[(1h-indol-3-yl)methyl]-1,9,12,15,18,21-hexaoxo-docosahydro-1h-pyrrolo[2,1-g]1,2-dithia-5,8,11,14,17,20-hexaazacyclotricosan-17-yl]acetic Acid
63. L-cysteinamide,n6-(aminoiminomethyl)-n2-(3-mercapto-1-oxopropyl)-l-lysylglycyl-l-
64. A-aspartyl-l-tryptophyl-l-prolyl-, Cyclic (1 Inverted Exclamation Marku6)-disulfide
65. N(sup 6)-amidino-n(sup 2)-(3-mercaptopropionyl)-l-lysylglycyl-l-alpha-aspartyl-l-tryptophyl-l-prolyl-l-cysteinamide, Cyclic(1-6)-disulphide
| Molecular Weight | 832.0 g/mol |
|---|---|
| Molecular Formula | C35H49N11O9S2 |
| XLogP3 | -2.4 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 10 |
| Exact Mass | 831.31561453 g/mol |
| Monoisotopic Mass | 831.31561453 g/mol |
| Topological Polar Surface Area | 377 Ų |
| Heavy Atom Count | 57 |
| Formal Charge | 0 |
| Complexity | 1520 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | EPTIFIBATIDE |
| Active Ingredient | EPTIFIBATIDE |
| Company | ACCORD HLTHCARE (Application Number: A205557); AKORN (Application Number: A204589); AMNEAL PHARMS (Application Number: A205581); AUROBINDO PHARMA LTD (Application Number: A206127); SAGENT PHARMS (Application Number: A204693); TEVA PHARMS USA (Application Number: A090854) |
| 2 of 2 | |
|---|---|
| Drug Name | INTEGRILIN |
| Active Ingredient | EPTIFIBATIDE |
| Company | SCHERING (Application Number: N020718) |
Platelet Aggregation Inhibitors
National Library of Medicine's Medical Subject Headings. Eptifibatide. Online file (MeSH, 2016). Available from, as of January 20, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Eptifibatide is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=Eptifibatide&Search=Search
Integrilin is indicated to decrease the rate of a combined endpoint of death or new myocardial infarction (MI) in patients with ACS (unstable angina (UA)/non-ST-elevation myocardial infarction (NSTEMI)), including patients who are to be managed medically and those undergoing percutaneous coronary intervention (PCI).
NIH; DailyMed. Current Medication Information for Integrilin (Eptifibatide) Injection, Solution (Updated: January 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5423624f-a2d8-4273-8c62-51839fccfd7e
Integrilin is indicated to decrease the rate of a combined endpoint of death, new myocardial infarction (MI), or need for urgent intervention in patients undergoing percutaneous coronary intervention (PCI), including those undergoing intracoronary stenting. /Included in US product label/
NIH; DailyMed. Current Medication Information for Integrilin (Eptifibatide) Injection, Solution (Updated: January 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5423624f-a2d8-4273-8c62-51839fccfd7e
Eptifibatide has been administered concomitantly with a thrombolytic agent (e.g., alteplase, tenecteplase) in a limited number of patients to prevent coronary artery reocclusion after an acute myocardial infarction. /NOT included in US product label/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1537
The most frequent and severe adverse effect of eptifibatide therapy is bleeding. Bleeding complications, which usually are minor and develop at vascular access (e.g., femoral puncture) sites (e.g., in patients undergoing percutaneous coronary intervention (PCI)), have been reported in 35-75% of patients receiving various dosages of eptifibatide in clinical studies. Bleeding is an extension of the pharmacologic action of eptifibatide and was classified in clinical trials principally according to criteria of the Thrombolysis in Myocardial Infarction (TIMI) study groups. Minor bleeding generally was defined as spontaneous gross hematuria or spontaneous hematemesis; observed blood loss with a decrease in hemoglobin concentration of 3-5 g/dL or a reduction in hematocrit of at least 10%; or a decrease of 4-5 g/dL or 12-15% in hemoglobin or hematocrit, respectively, with no identifiable bleeding site. Major bleeding was defined as intracranial hemorrhage or overt bleeding associated with a hemoglobin or hematocrit decrease of at least 5 g/dL or at least 15%, respectively.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1538
Because eptifibatide increases the risk of bleeding, the drug is contraindicated in patients with a history of bleeding diathesis or active abnormal bleeding (e.g., elevated hemostatic indices, recent noncompressible vascular punctures GI or genitourinary bleeding) within the previous 30 days. A low hematocrit value (less than 30%) at baseline could represent recent undetected bleeding, and patients with such values may not be able to tolerate additional bleeding episodes; eptifibatide should not be used in these patients. Eptifibatide also is contraindicated in patients with severe uncontrolled hypertension (systolic blood pressure exceeding 200 mm Hg or diastolic blood pressure exceeding 110 mm Hg with antihypertensive therapy); recent (within 6 weeks) major surgery; history of stroke within 30 days or any history of hemorrhagic stroke; current or planned therapy with another GP IIb/IIIa-receptor inhibitor; and patients receiving renal dialysis. No data are available on the use of eptifibatide in patients with serum creatinine concentrations of 4 mg/dL or greater; the dosage should be reduced in patients with serum creatinine concentrations between 2-4 mg/dL.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1539
FDA Pregnancy Risk Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./
NIH; DailyMed. Current Medication Information for Integrilin (Eptifibatide) Injection, Solution (Updated: January 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5423624f-a2d8-4273-8c62-51839fccfd7e
Safety and effectiveness of Integrilin in pediatric patients have not been studied.
NIH; DailyMed. Current Medication Information for Integrilin (Eptifibatide) Injection, Solution (Updated: January 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5423624f-a2d8-4273-8c62-51839fccfd7e
For more Drug Warnings (Complete) data for Eptifibatide (15 total), please visit the HSDB record page.
For treatment of myocardial infarction and acute coronary syndrome.
FDA Label
Integrilin is intended for use with acetylsalicylic acid and unfractionated heparin.
Integrilin is indicated for the prevention of early myocardial infarction in patients presenting with unstable angina or non-Q-wave myocardial infarction with the last episode of chest pain occurring within 24 hours and with ECG changes and / or elevated cardiac enzymes.
Patients most likely to benefit from Integrilin treatment are those at high risk of developing myocardial infarction within the first 3-4 days after onset of acute angina symptoms including for instance those that are likely to undergo an early percutaneous transluminal coronary angioplasty (PTCA).
Eptifibatide is an anti-coagulant that selectively and reversibly blocks the platelet glycoprotein IIb/IIIa receptor.
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
B01AC16
B01AC16
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC16 - Eptifibatide
Clearance
55 mL/kg/h [patients with coronary artery disease]
/MILK/ It is not known whether eptifibatide is distributed into milk in humans.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1541
Eptifibatide is approximately 25% bound to plasma proteins, principally (9-16%) to albumin.The volume of distribution of eptifibatide in patients with coronary artery disease is about 185-260 mL/kg and is somewhat higher (220-270 mL/kg) in healthy individuals.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1541
Eptifibatide, a synthetic peptide inhibitor of the platelet glycoprotein IIb/IIIa receptor, has been studied as an antithrombotic agent in a variety of acute ischemic coronary syndromes. The purpose of the present study was to characterize the disposition of (14)C-eptifibatide in man after a single intravenous (i.v.) bolus dose. (14)C-Eptifibatide (approximately 50 uCi) was administered to eight healthy men as a single 135-ug/kg IV bolus. Blood, breath carbon dioxide, urine, and fecal samples were collected for up to 72 hours postdose and analyzed for radioactivity by liquid scintillation spectrometry. Plasma and urine samples were also assayed by liquid chromatography with mass spectrometry for eptifibatide and deamidated eptifibatide (DE). Mean (+/- SD) peak plasma eptifibatide concentrations of 879 +/- 251 ng/mL were achieved at the first sampling time (5 minutes), and concentrations then generally declined biexponentially, with a mean distribution half-life of 5 +/- 2.5 minutes and a mean terminal elimination half-life of 1.13 +/- 0.17 hours. Plasma eptifibatide concentrations and radioactivity declined in parallel, with most of the radioactivity (82.4%) attributed to eptifibatide. A total of approximately 73% of administered radioactivity was recovered in the 72-hour period following (14)C-eptifibatide dosing. The primary route of elimination was urinary (98% of the total recovered radioactivity), whereas fecal (1.5%) and breath (0.8%) excretion was small. Eptifibatide is cleared by both renal and nonrenal mechanisms, with renal clearance accounting for approximately 40% of total body clearance. Within the first 24 hours, the drug is primarily excreted in the urine as unmodified eptifibatide (34%), DE (19%), and more polar metabolites (13%).
PMID:9589822 Alton KB et al; Clin Ther 20 (2): 307-23 (1998)
Plasma clearance of eptifibatide is proportional to body weight and estimated creatinine clearance and inversely proportional to age. Following a single IV dose of (14)C-radiolabeled eptifibatide (135 ug/kg) in healthy men, renal clearance averaged approximately 40-50% of total body clearance. Clearance is reduced by 50% in patients with moderate to severe renal impairment (estimated Clcr less than 50 mL/minute). Total body clearance in geriatric patients with coronary artery disease is lower than that in younger adults.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1541
For more Absorption, Distribution and Excretion (Complete) data for Eptifibatide (9 total), please visit the HSDB record page.
No major metabolites have been detected in human plasma. Deamidated eptifibatide and other, more polar metabolites have been detected in urine.
(14)C-eptifibatide was extensively metabolized to deamidated eptifibatide and to several polar metabolites by both rats and monkeys. The drug-derived radioactivity excreted into the bile by rats, and identified as deamidated eptifibatide, was reabsorbed from the intestinal tract and further metabolized to more polar metabolites. The plasma and urine metabolite profiles in rats and monkeys indicate that the metabolic disposition of eptifibatide is similar for the two species.
Health Canada; Product Monograph for Integrilin (Eptifibatide Injection), Drug Identification Number (DIN): 02240351 p.25 (Date of Preparation: February 16, 2011).
Eptifibatide is metabolized principally through deamidation to a metabolite that has approximately 41% of the platelet-aggregation inhibitory activity of the parent compound, and through formation of other more polar metabolites. Approximately 27% of a dose of eptifibatide is broken down in plasma into naturally occurring amino acids; no major non-amino acid metabolites have been detected in plasma in humans.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1541
Approximately 2.5 hours
In Cynomolgus monkeys, plasma concentrations of (14)C-eptifibatide-derived radioactivity declined with a half life of about 12 hours following a single 2 mg/kg IV dose of (14)C-eptifibatide. Unchanged eptifibatide, which accounted for approximately 93% of total plasma (14)C at 5 min post-dose, was eliminated rapidly with a half life of 17 min.
Health Canada; Product Monograph for Integrilin (Eptifibatide Injection), Drug Identification Number (DIN): 02240351 p.24 (Date of Preparation: February 16, 2011). Available from, as of March 8, 2016: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
In rats, plasma concentrations of (14)C-eptifibatide-derived radioactivity declined rapidly with a terminal phase half life of about 5 hours following a single 2 mg/kg IV dose of the radiolabeled drug. Unchanged eptifibatide declined with an apparent half life of about 8 min. Following single 2 and 20 mg/kg IV doses, the plasma concentrations of eptifibatide were dose-proportional and the half life (11 to 12 min) was dose-independent, indicating linear kinetics within the 2 to 20 mg/kg dose range.
Health Canada; Product Monograph for Integrilin (Eptifibatide Injection), Drug Identification Number (DIN): 02240351 p.24 (Date of Preparation: February 16, 2011). Available from, as of March 8, 2016: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
The half-life of eptifibatide in patients with coronary artery disease averages 2.5-2.8 hours. In healthy individuals, half-life of the drug reportedly averages 0.83-2.4 hours.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1541
... The purpose of the present study was to characterize the disposition of (14)C-eptifibatide in man after a single intravenous (IV) bolus dose. (14)C-Eptifibatide (approximately 50 uCi) was administered to eight healthy men as a single 135-ug/kg IV bolus. ... Mean (+/- SD) peak plasma eptifibatide concentrations of 879 +/- 251 ng/mL were achieved at the first sampling time (5 minutes), and concentrations then generally declined biexponentially, with a mean distribution half-life of 5 +/- 2.5 minutes and a mean terminal elimination half-life of 1.13 +/- 0.17 hours. ...
PMID:9589822 Alton KB et al; Clin Ther 20 (2): 307-23 (1998)
Eptifibatide inhibits platelet aggregation by reversibly binding to the platelet receptor glycoprotein (GP) IIb/IIIa of human platelets, thus preventing the binding of fibrinogen, von Willebrand factor, and other adhesive ligands. Inhibition of platelet aggregation occurs in a dose- and concentration-dependent manner.