

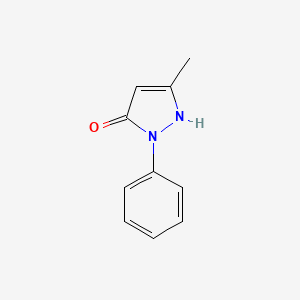

1. N-demethylantipyrine
2. N-demethylantipyrine, 14c-labeled
1. 19735-89-8
2. 5-methyl-2-phenyl-1,2-dihydropyrazol-3-one
3. 942-32-5
4. N-demethylantipyrine
5. 5-methyl-2-phenyl-1h-pyrazol-3(2h)-one
6. 5-methyl-2-phenyl-2h-pyrazol-3-ol
7. 5-methyl-2-phenyl-1h-pyrazol-3-one
8. 1,2-dihydro-5-methyl-2-phenyl-3h-pyrazol-3-one
9. 5-hydroxy-3-methyl-1-phenylpyrazole
10. Demethylated Antipyrine
11. 3h-pyrazol-3-one, 1,2-dihydro-5-methyl-2-phenyl-
12. 1h-pyrazol-5-ol, 3-methyl-1-phenyl-
13. 5-methyl-2-phenyl-1,2-dihydro-3h-pyrazol-3-one
14. 3-methyl-1-phenylpyrazolin-5-one
15. 5-methyl-2-phenyl-2,3-dihydro-1h-pyrazol-3-one
16. 5-methyl-2-phenyl-1,2- Dihydro-3h-pyrazol-3-one (3)
17. Edarabone
18. Edaravone [inn]
19. Einecs 213-390-4
20. Einecs 243-261-8
21. Edaravone [jan]
22. Edaravone [hsdb]
23. Edaravone [usan]
24. 1-phenyl-3-methyl-1h-pyrazole-5(2h)-one
25. Norphenazone [mi]
26. Edaravone [mart.]
27. Cambridge Id 5118906
28. Cid_4021
29. Edaravone [who-dd]
30. Schembl34121
31. Schembl200831
32. 3h-pyrazol-3-one,1,2-dihydro-5-methyl-2-phenyl-
33. Edaravone [orange Book]
34. Chembl4303694
35. Dtxsid6091550
36. Dtxsid9061334
37. 5-methyl-2-phenylpyrazol-3-one
38. Bdbm38550
39. 1-phenyl-3-methyl-5-oxopyrazole
40. Chebi:190738
41. 1-phenyl-3-methyl-5-pyrazolinone
42. 1-phenyl-3-methyl-pyrazol-5-one
43. 1-phenyl-3-methylpyrazolin-5-one
44. Hms1577a16
45. 3-methyl-1-phenyl-pyrazole-5-one
46. Amy12227
47. 1-phenyl-3-methyl-5-hydroxypyrazole
48. Bbl038006
49. Mfcd00127930
50. Mfcd00462237
51. Stk062417
52. Stk398253
53. 2-phenyl-5-methyl-3h-pyrazol-3-one
54. 3-methyl-1-phenyl-1h-pyrazol-5-one
55. 5-methyl-2-phenyl-2-pyrazolin-3-one
56. Akos000309891
57. Akos000313062
58. Zinc100006441
59. Zinc100026603
60. 16,16-dimethylprostaglandind2
61. Phenyl Methyl Pyrazolone [inci]
62. 5-hydroxy-3-methyl-1-phenyl-1h-pyrazole
63. Da-00482
64. Phenazone Impurity A [ep Impurity]
65. 5-methyl-2-phenyl-2h-pyrazol-3(4h)-one
66. Db-014594
67. Cs-0172114
68. Cs-0319008
69. Ft-0649724
70. Ft-0701986
71. 3-methyl-5-oxo-1-phenyl-2-pyrazoline-4-ide
72. 3-methyl-1-phenyl-4,5-dihydropyrazole-5-one
73. 5-methyl-2-phenyl-1,2-dihydro-pyrazol-3-one
74. Antipyrine Related Compound A [usp-rs]
75. F20192
76. 1-phenyl-3-methyl-1h-4,5-dihydropyrazol-5-one
77. 735m898
78. Antipyrine Related Compound A [usp Impurity]
79. 3h-pyrazol-3-one, 2,4-dihydro-5-methyl-2-phenyl
80. 1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-4-carboxylicacid
| Molecular Weight | 174.20 g/mol |
|---|---|
| Molecular Formula | C10H10N2O |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 174.079312947 g/mol |
| Monoisotopic Mass | 174.079312947 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 241 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Edaravone has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-(5-methyl-2-phenylpyrazol-3-yl)oxyoxane-2-carboxylic acid.
Norantipyrine is a known human metabolite of antipyrine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560