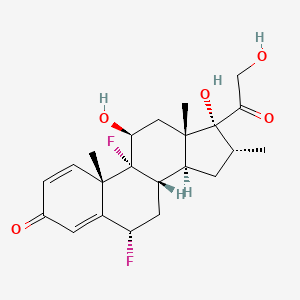

1. Fluorodexamethasone
1. Flumetasone
2. 2135-17-3
3. Aniprime
4. Flucorticin
5. Flumethason
6. Anaprime
7. Methagon
8. Fluvet
9. Cortexilar
10. Flumetasona
11. Flumetasonum
12. Rs 2177
13. Flumetasone [inn]
14. Rs-2177
15. Flumethasone [usan]
16. 6alpha,9-difluoro-11beta,17,21-trihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione
17. Nsc-54702
18. 6alpha,9alpha-difluoro-16alpha-methylprednisolone
19. U-10974
20. U-10,974
21. Lr3cd8sx89
22. Mls000028618
23. Chebi:34764
24. Flumetasone (inn)
25. (6s,8s,9r,10s,11s,13s,14s,16r,17r)-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
26. Flumethasone (usan)
27. Smr000058690
28. Dsstox_cid_25365
29. Dsstox_rid_80828
30. Pregna-1,4-diene-3,20-dione,6,9-difluoro-11,17,21-trihydroxy-16-methyl-, (6a,11b,16a)-
31. Dsstox_gsid_45365
32. Flumethasonum
33. 6alpha-fluorodexamethasone
34. Mfcd00056464
35. Flucort (veterinary)
36. 6.alpha.-fluorodexamethasone
37. Nsc 54702
38. Flumetasonum [inn-latin]
39. 6-alpha-fluorodexamethasone
40. Flumetasona [inn-spanish]
41. Nsc54702
42. Ncgc00016604-01
43. Prestwick_229
44. Cas-2135-17-3
45. Einecs 218-370-9
46. Brn 5645455
47. Opera_id_1501
48. Prestwick0_000734
49. Prestwick1_000734
50. Prestwick2_000734
51. Prestwick3_000734
52. Flumethasone [mi]
53. Unii-lr3cd8sx89
54. 6alpha-fluoro-dexamethasone
55. Schembl4046
56. Bspbio_000688
57. Flumetasone [who-dd]
58. Mls001077306
59. Spbio_002627
60. Bpbio1_000758
61. 6,9-difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
62. Chembl1201392
63. Dtxsid2045365
64. Flumethasone [green Book]
65. Hms1570c10
66. Hms2097c10
67. Hms2234b11
68. Hms3714c10
69. (6s,8s,9r,10s,11s,13s,14s,16r,17r)-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
70. Amy38487
71. Hy-b1051
72. Pregna-1,4-diene-3,20-dione, 6,9-difluoro-11,17,21-trihydroxy-16-methyl-, (6.alpha.,11.beta.,16.alpha.)-
73. Zinc3983936
74. Tox21_110518
75. S4088
76. Akos005256462
77. Akos015895435
78. Tox21_110518_1
79. Ac-2069
80. Ccg-220734
81. Cs-4573
82. Db00663
83. Flumethasone 100 Microg/ml In Methanol
84. Smp1_000127
85. Ncgc00021761-03
86. Ncgc00021761-05
87. Ncgc00021761-06
88. (6alpha,11beta,16alpha)-6,9-difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
89. As-12534
90. Pregna-1,4-diene-3,20-dione, 6alpha,9-difluoro-11beta,17,21-trihydroxy-16alpha-methyl-(van)
91. Flumethasone 100 Microg/ml In Acetonitrile
92. F0945
93. D04208
94. Ec 218-370-9
95. T72075
96. 135f173
97. A855571
98. Sr-01000003144
99. Q-101387
100. Q4383636
101. Sr-01000003144-3
102. Brd-k61496577-001-03-8
103. Brd-k61496577-001-20-2
104. 6.alpha., 9.alpha.-difluoro-16.alpha.-methylprednisolone
105. (6?,11?,16?)-6,9-difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione
106. (1r,2s,8s,10s,11s,13r,14r,15s,17s)-1,8-difluoro-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,13,15-trimethyltetracyclo[8.7.0.0?,?.0??,??]heptadeca-3,6-dien-5-one
107. (6s,9r,10s,11s,13s,16r,17r)-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxy-acetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-cyclopenta[a]phenanthren-3-one
108. 6.alpha., 9-difluoro-11.beta.,17,21-trihydroxy-16.alpha.-methylpregna-1, 4-diene-3,20-dione
109. 6.alpha.,9-difluoro-11.beta.,17,21-trihydroxy-16.alpha.-methylpregna-1,4-diene-3,20-dione
110. 6alpha,9-difluoro-11beta,17,21-trihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione (flumetasone)
111. Pregna-1,4-diene-3,20-dione, 6,9-difluoro-11,17, 21-trihydroxy-16-methyl-, (6.alpha.,11.beta.,16.alpha.)-
112. Pregna-1,4-diene-3,20-dione, 6.alpha.,9-difluoro-11.beta.,17,21-trihydroxy-16.alpha.-methyl-
| Molecular Weight | 410.5 g/mol |
|---|---|
| Molecular Formula | C22H28F2O5 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 410.19048031 g/mol |
| Monoisotopic Mass | 410.19048031 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 839 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of contact dermatitis, atopic dermatitis, exczema, psoriasis, diaper rash and other skin conditions
Flumethasone pivalate is a moderately potent difluorinated corticosteroid ester with anti-inflammatory, antipruritic and vasoconstrictive properties. As it is a privalate salt, its anti-inflammatory action is concentrated at the site of application. This local effect on diseased areas results in a prompt decrease in inflammation, exudation and itching.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AB - Corticosteroids, moderately potent (group ii)
D07AB03 - Flumetasone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07X - Corticosteroids, other combinations
D07XB - Corticosteroids, moderately potent, other combinations
D07XB01 - Flumetasone
Absorption
Minimal if applied topically
Primarily hepatic
Flumethasone is a glucocorticoid receptor agonist. This complex binds to the nucleus causing a variety of genetic activation and repressions. The antiinflammatory actions of corticosteroids are thought to involve lipocortins, phospholipase A2 inhibitory proteins which, through inhibition arachidonic acid, control the biosynthesis of prostaglandins and leukotrienes. The immune system is suppressed by corticosteroids due to a decrease in the function of the lymphatic system, a reduction in immunoglobulin and complement concentrations, the precipitation of lymphocytopenia, and interference with antigen-antibody binding. Flumethasone binds to plasma transcortin, and it becomes active when it is not bound to transcortin.