
1. (+)-norpseudoephedrine
2. Benzenemethanol, Alpha-((1r)-1-aminoethyl)-, (alphar)-rel-
3. Cathine
4. Cathine Hydrochloride
5. Exponcit
6. Fasupond
7. Fugoa Depot
8. Norpseudoephedrine Hydrobromide
9. Norpseudoephedrine Hydrochloride
10. Norpseudoephedrine Hydrochloride, (+)-isomer
11. Norpseudoephedrine Hydrochloride, (r*,s*)-(+-)-isomer
12. Norpseudoephedrine Hydrochloride, (r*,s*)-isomer
13. Norpseudoephedrine Hydrochloride, (r-(r*,r*))-isomer
14. Norpseudoephedrine Hydrochloride, (s-(r*,s*))-isomer
15. Norpseudoephedrine Sulfate (2:1), (+)-isomer
16. Norpseudoephedrine Sulfate (2:1), (r-(r*,r*))-isomer
17. Norpseudoephedrine Sulfate, (r-(r*,s*))-isomer
18. Norpseudoephedrine Sulfate, (s-(r*,r*))-isomer
19. Norpseudoephedrine Tartrate, (s-(r*,r*))-(r-(r*,r*))-isomer
20. Norpseudoephedrine, (-)-isomer
21. Norpseudoephedrine, (r*,r*)-(+-)-isomer
22. Norpseudoephedrine, (r*,s*)-isomer
23. Norpseudoephedrine, (r-(r*,s*))-isomer
24. Norpseudoephedrine, (s-(r*,r*))-isomer
25. Norpseudoephedrine, (s-(r*,s*))-isomer
26. Norpseudoephedrine, Conjugate Monoacid, (r-(r*,s*))-isomer
27. Pseudonorephedrine
1. (-)-norpseudoephedrine
2. (1r,2r)-2-amino-1-phenylpropan-1-ol
3. 37577-07-4
4. Norpseudoephedrine, (-)-
5. Qq0fvc4pxs
6. L-nor-psi-ephedrine
7. Fugoa
8. Chebi:8104
9. Chembl1788114
10. L-nor-psi-ephedrin [german]
11. (1r,2r)-2-amino-1-phenyl-propan-1-ol
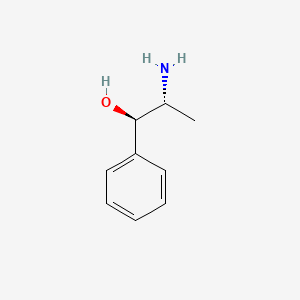
12. Phenylpropanolamine
13. Beta-hydroxyamphetamine
14. L-pseudonorephedrine
15. L-norpseudoephedrine
16. Unii-qq0fvc4pxs
17. (-)-pseudo Norephedrine
18. 2-amino-1-phenyl-1-propanol #
19. (r,r)-(-)-norpseudoephedrine
20. Prestwick0_000324
21. Prestwick1_000324
22. Prestwick2_000324
23. (-)-threo-2-amino-2-methyl-1-phenylethanol
24. (r-(r*,r*))-alpha-(1-aminoethyl)benzenemethanol
25. Norpseudoephedrine, L-
26. Schembl125333
27. Spbio_002248
28. Benzenemethanol, .alpha.-((1r)-1-aminoethyl)-, (.alpha.r)-
29. Benzenemethanol, Alpha-((1r)-1-aminoethyl)-, (alphar)-
30. Benzenemethanol, .alpha.-(1-aminoethyl)-, [r-(r*,r*)]-
31. Dtxsid001045718
32. (1r,2r)-pseudonorephedrine
33. 14838-15-4
34. Bdbm50367603
35. Pdsp1_001354
36. Pdsp2_001338
37. Pd017909
38. (r*,r*)-alpha-(1-aminoethyl)benzylalcohol
39. Ns00098695
40. Q6456100
41. (1r,2r)-(-)-norpseudoephedrine, >=98.0% (nt)
42. [r-(r*,r*)]-.alpha.-(1-aminoethyl)benzenemethanol
| Molecular Weight | 151.21 g/mol |
|---|---|
| Molecular Formula | C9H13NO |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 46.2 |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 110 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic alpha-Agonists; Adrenergic Agents; Appetite Depressants; Nasal Decongestants; Sympathomimetics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
The Food and Drug Administration (FDA) is issuing a public health advisory concerning phenylpropanolamine hydrochloride. This drug is widely used as a nasal decongestant (in over-the-counter and prescription drug products) and for weight control (in over-the-counter drug products). FDA is taking steps to remove phenylpropanolamine from all drug products and has requested that all drug companies discontinue marketing products containing phenylpropanolamine. Phenylpropanolamine has been marketed for many years. A recent study reported that taking phenylpropanolamine increases the risk of hemorrhagic stroke (bleeding into the brain or into tissue surrounding the brain) in women. Men may also be at risk. Although the risk of hemorrhagic stroke is very low, FDA recommends that consumers not use any products that contain phenylpropanolamine.
FDA, Center for Drug Evaluation and Research (CDER); Food and Drug Administration Public Health Advisory Subject: Safety of Phenylpropanolamine (November 6, 2000) Available from, as of March 14, 2007: https://www.fda.gov/cder/drug/infopage/ppa/advisory.htm
/Experimental/ Pregnancy rhinitis is common and very troublesome for many women. Today, no safe and effective treatment is available for this condition. The aim of this placebo-controlled double-blind study was to evaluate the decongestive effect of phenylpropanolamine (PPA 50 mg) twice daily for seven days in 38 women with pregnancy rhinitis. In the morning, before starting the course of treatment, and two to three hours after taking the last dose of the study-medicine in the morning on the eighth day, recordings of the position of the nasal mucosal surface were made with rhinostereometry. Every evening, the women filled in a questionnaire about their symptoms on a scale from 0-9 (0 = no symptoms, 9 = extremely severe symptoms). The effects of the drug on their blood pressure and other side-effects were also determined. The patients used a newly-evaluated telephone method to assess their daily symptoms. PPA 50 mg had a decongestive effect on the nasal mucosa, as measured with symptom scores and rhinostereometry. In the placebo group, this effect was found with rhinostereometry, but not on nasal stuffiness as judged by the symptom scores. The reason why the placebo group also experienced a decongestive effect after treatment may have been due to stress because the patients were in a hurry and such stress may have a decongestive effect on the nasal mucosa. No effects on the blood pressure or other side-effects were detected. In conclusion, this study shows that PPA 50 mg twice daily may be an effective and safe treatment in pregnancy rhinitis.
PMID:17216745 Graf P et al ; Rhinology 44 (4): 274-7 (2006)
VET: A six-month-old kitten had congenital urethral sphincter mechanism incompetence due to urethral hypoplasia and associated uterine hypoplasia and vaginal aplasia. Diagnosis was based on radiographic examination, surgical exploration and histological examination of the lower urinary tract. Surgical correction resulted in a marked clinical improvement. The cat became fully continent following treatment with phenylpropanolamine.
PMID:10404490 Baines SJ et al; J Small Anim Pract 40 (6): 286-90 (1999)
For more Therapeutic Uses (Complete) data for PHENYLPROPANOLAMINE (6 total), please visit the HSDB record page.
The Food and Drug Administration (FDA) is issuing a public health advisory concerning phenylpropanolamine hydrochloride. This drug is widely used as a nasal decongestant (in over-the-counter and prescription drug products) and for weight control (in over-the-counter drug products). FDA is taking steps to remove phenylpropanolamine from all drug products and has requested that all drug companies discontinue marketing products containing phenylpropanolamine. Phenylpropanolamine has been marketed for many years. A recent study reported that taking phenylpropanolamine increases the risk of hemorrhagic stroke (bleeding into the brain or into tissue surrounding the brain) in women. Men may also be at risk. Although the risk of hemorrhagic stroke is very low, FDA recommends that consumers not use any products that contain phenylpropanolamine.
FDA, Center for Drug Evaluation and Research (CDER); Food and Drug Administration Public Health Advisory Subject: Safety of Phenylpropanolamine (November 6, 2000) Available from, as of March 14, 2007: https://www.fda.gov/cder/drug/infopage/ppa/advisory.htm
Several reports have linked the abuse of phenylpropanolamine with myocardial injury, especially when overdose is involved. ... The first case of phenylpropanolamine induced myocardial injury in a young woman who was using it at recommended doses for weight control /is presented/.
PMID:12734532 Pilsczek FH et al; Heart Lung 32 (2): 100-4 (2003)
Evidence shows that post partum women may be at greater risk than the rest of the population of developing psychiatric disorders with the use of phenylpropanolamine at recommended doses and with overdose.
USP. Convention. USPDI - Drug Information for the Health Care Professional. 19th ed. Volume I.Micromedex, Inc. Englewood, CO., 1999. Content Prepared by the U.S. Pharmacopieal Convention, Inc., p. 2314
Although urinary retention and increased intraocular pressure may be associated with ephedrine, these adverse effects have not been observed in patients receiving therapeutic dosages of phenylpropanolamine. However, the drug should be used with caution in patients with glaucoma or prostatic hypertrophy. Phenylpropanolamine should be used with caution in patients with hyperthyroidism, cardiovascular disorders, hypertension, or diabetes mellitus. Patients should be instructed to discontinue phenylpropanolamine and consult their physician if nervousness, dizziness, or insomnia occurs. /Phenylpropanolamine hydrochloride/
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1063
For more Drug Warnings (Complete) data for PHENYLPROPANOLAMINE (13 total), please visit the HSDB record page.
/In adults:/ toxic dose /is 100 mg/ (although CNS and cardiovascular hyperadrenergic signs such as sweating and skin flushing may occur at a lower dose).
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 515
For the treatment of nasal congestion, control of urinary incontinence, priapism and obesity.
Appetite Depressants
Agents that are used to suppress appetite. (See all compounds classified as Appetite Depressants.)
R - Respiratory system
R01 - Nasal preparations
R01B - Nasal decongestants for systemic use
R01BA - Sympathomimetics
R01BA01 - Phenylpropanolamine
Absorption
Reduced bioavailability (about 38%) from gastrointestinal tract because of first pass metabolism by monoamine oxidase in the stomach and liver.
Phenylpropanolamine is well absorbed orally, and the peak blood concentration is attained within one to two hours. ... The drug is principally excreted unchanged; the major route of excretion is renal. Since phenylpropanolamine is a weak base, elimination is enhanced in acid urine.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 2002
Phenylpropanolamine is readily absorbed from the GI tract. Nasal decongestion reportedly occurs within 15-30 minutes after oral administration of 25 mg of phenylpropanolamine hydrochloride and appears to persist for 3 hours. Plasma concentrations of the drug required for a therapeutic effect are not known. In one study, peak plasma concentrations of 100 ng/mL were reached in 1-2 hours and concentrations remained greater than 60 ng/ml for 6 hours following oral administration of 50 mg of phenylpropanolamine hydrochloride to fasting adults. Following administration of 150 mg of an extended-release preparation of the drug, peak plasma concentrations of 300 ng/mL occurred after 3.5 hours and phenylpropanolamine concentrations remained greater than 180 ng/mL for 12 hours. /Phenylpropanolamine hydrochloride/
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1062
Animal studies indicate that phenylpropanolamine is distributed into various tissues and fluids, including /cerebrospinal fluid/ and brain. /Phenylpropanolamine hydrochloride/
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1063
The bioavailability and pharmacokinetics of phenylpropanolamine hydrochloride from a controlled release caplet and solution were studied in 12 male subjects, aged 18 to 36 yr, who received either a 75 mg caplet once daily or a 25 mg solution 3 times daily for 4 days. Maximum plasma concentrations, time to maximum concentration, and areas under the concentration-time curves are reported for both caplet and solution. The mean first order absorption rate constant, elimination half-life and lag time for the drug from the caplet were 0.488 ngxhr/mL, 5.84 hr, and 0.394 hr, respectively, and 2.87 ngxhr/mL, 3.73 hr, and 0.325 hr, respectively, from the solution. The smaller apparent mean first order absorption rate constant and longer elimination half-life from the caplet is due to the slow release of drug, thereby slowing its absorption and producing sustained plasma drug concentrations.
PMID:2265237 Shargel L et al; Biopharm Drug Dispos 11 (Oct): 569-83 (1990)
For more Absorption, Distribution and Excretion (Complete) data for PHENYLPROPANOLAMINE (6 total), please visit the HSDB record page.
Hepatic
Like other phenylisopropanolamines, small amounts of the drug are slowly metabolized in the liver to an active hydroxylated metabolite. About 80-90% of a dose of phenylpropanolamine is excreted unchanged in the urine within 24 hours. /Phenylpropanolamine hydrochloride/
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1063
2.1 to 3.4 hours.
The half-life ranges from 2.7 to 3.4 hrs. /Phenylpropanolamine hydrochloride/
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 2002
Phenylpropanolamine reportedly has a half-life of 3-4 hours. /Phenylpropanolamine hydrochloride/
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1063
The bioavailability and pharmacokinetics of phenylpropanolamine hydrochloride (PPA HCl) from a controlled-release (CR) caplet and solution was studied in 12 male subjects, who received either a 75 mg PPA HCl CR caplet once daily or a 25 mg PPA HCl solution given three times a day. All subjects received the medication for 4 consecutive days. ... The mean elimination half-life for PPA HCl from the CR caplet was 5.84 hr and 3.73 hr, for the solution. ...
PMID:2265237 Shargel L et al; Biopharm Drug Dispos 11 (7): 569-83 (1990)
Phenylpropanolamine acts directly on alpha- and, to a lesser degree, beta-adrenergic receptors in the mucosa of the respiratory tract. Stimulation of alpha-adrenergic receptors produces vasoconstriction, reduces tissue hyperemia, edema, and nasal congestion, and increases nasal airway patency. PPA indirectly stimulates beta-receptors, producing tachycardia and a positive inotropic effect.
The mechanism of action of phenylpropanolamine has not been conclusively determined. The drug may directly stimulate adrenergic receptors but probably indirectly stimulates both A- and B-adrenergic receptors by releasing norepinephrine from its storage sites. It is believed that B-adrenergic effects result from stimulation of cyclic adenosine 3',5'-monophosphate (AMP) production by activation of the enzyme adenyl cyclase, whereas A-adrenergic effects result from inhibition of adenyl cyclase activity. With prolonged use or too frequent administration, indirectly acting sympathomimetics may deplete norepinephrine in sympathetic nerve endings and tachyphylaxis may develop. Tachyphylaxis induced by one indirectly acting sympathomimetic may result in refractoriness to other drugs of the same class. /Phenylpropanolamine hydrochloride/
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1062
Whether the alpha1- and alpha2-adrenoceptor mediated increases in diastolic blood pressure effected by phenylpropanolamine and its enantiomers are altered by, or independent of beta2-mediated vasodilation, or beta2-adrenoceptor blockade were studied in pithed rats. The pressor responses were enhanced in the presence of the antagonist ICI-118551 and diminished in the presence of albuterol. It was concluded that each form of phenylpropanolamine possesses the intrinsic ability to interact with all of the adrenoceptors in the system used and that the interaction with those adrenoceptors determines the net increase in diastolic blood pressure that follows the intravenous administration of the compounds. These findings have a bearing on the recent controversy regarding the use of beta-blocking agents in the treatment of overdosage of phenylpropanolamine.
Moya-Huff FA, Maher TJ; J Pharm Pharmacol 40 (Dec) 876-8 (1988)