
1. Gs-7340
2. Gs-734003
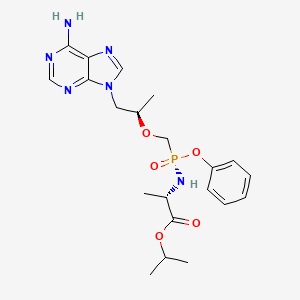
3. L-alanine, N-((s)-(((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)phenoxyphosphinyl)-, 1-methylethyl Ester
4. Vemlidy
1. 379270-37-8
2. Gs-7340
3. Gs 7340
4. Unii-el9943ag5j
5. Gs-734003
6. El9943ag5j
7. 383365-04-6
8. Vemlidy
9. L-alanine, N-((s)-(((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)phenoxyphosphinyl)-, 1-methylethyl Ester
10. (s)-isopropyl 2-(((s)-((((r)-1-(6-amino-9h-purin-9-yl)propan-2-yl)oxy)methyl)(phenoxy)phosphoryl)amino)propanoate
11. Propan-2-yl (2s)-2-[[[(2r)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethyl-phenoxyphosphoryl]amino]propanoate
12. Gs7340
13. J4414g3buk
14. Tenofovir Alafenamide [usan]
15. Taf
16. Gs-7339
17. Tenofovir Alafenamide [usan:inn]
18. L-alanine, N-[(s)-[[(1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl Ester
19. Unii-j4414g3buk
20. Schembl3107149
21. Chembl2107825
22. Gs-7340 Tenofovir Alafenamide
23. Chebi:90926
24. Dtxsid50958941
25. Ex-a610
26. Gs-7340; Tenofovir Alafenamide
27. Tenofovir Alafenamide (usan/inn)
28. Tenofovir Alafenamide [mi]
29. Tenofovir Alafenamide [inn]
30. Isopropyl (2s)-2-[[[(1r)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-phenoxy-phosphoryl]amino]propanoate
31. L-alanine, N-((r)-(((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)phenoxyphosphinyl)-, 1-methylethyl Ester
32. N-((r)-(((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)phenoxyphosphinyl)-l-alanine 1-methylethyl Ester
33. Mfcd23843796
34. Akos016009341
35. Tenofovir Alafenamide [who-dd]
36. Zinc100055899
37. Ccg-269506
38. Cs-3366
39. Db09299
40. Ncgc00390564-03
41. Ncgc00390564-04
42. Ac-29893
43. As-55942
44. Hy-15232
45. B8021
46. S7856
47. Tenofovir Alafenamide (prodrug Of Tenofovir)
48. D10428
49. Q22075912
50. Sp-tenofovir-phosphonamidate, Phenyl, L-alanine Isopropyl Ester
51. (s)-isopropyl 2-((s)-(((r)-1-(6-amino-9h-purin-9-yl)propan-2-yloxy)methyl)(phenoxy)phosphorylamino)propanoate
52. 1-methylethyl N-((s)-(((1r)-2-(6-amino-9h-purin-9-yl)-1-methylethoxy)methyl)phenoxyphosphinyl)-l-alaninate
53. 9-[(r)-2-[[(s)-[[(s)-1-(isopropoxycarbonyl)ethyl]amino]phenoxyphosphinyl]methoxy]propyl]adenine
54. L-alanine, Sp-n-[[[[(1r)-2-(6-amino-9h-purin-9-yl)-1-methylethyl]oxy]methyl]phenoxyphosphinyl]-, Isopropyl Ester
55. Propan-2-yl (2s)-2-{[(s)-({[(2r)-1-(6-amino-9h-purin-9-yl)propan-2-yl]oxy}methyl)(phenoxy)phosphoryl]amino}propanoate
56. Propan-2-yl N-[(s)-({[(2r)-1-(6-amino-9h-purin-9-yl)propan-2-yl]oxy}methyl)(phenoxy)phosphoryl]-l-alaninate
| Molecular Weight | 476.5 g/mol |
|---|---|
| Molecular Formula | C21H29N6O5P |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 476.19370504 g/mol |
| Monoisotopic Mass | 476.19370504 g/mol |
| Topological Polar Surface Area | 144 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 680 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tenofovir alafenamide is indicated for the treatment of chronic hepatitis B in adult patients with compensated liver disease. In combination with [emtricitabine] and other antiretrovirals, it is indicated for the treatment of HIV-1 infection in adolescent and adult patients with a weight higher than 35 kg. This combination is also indicated to prevent HIV-1 infections in high risk adolescent and adult patients, excluding patients at risk from receptive vaginal sex. When combined with antiretrovirals other than protease inhibitors that require a CYP3A inhibitor, it can be used to treat pediatric patients weighing 25-35 kg. In the combination product with emtricitabine and [bictegravir], tenofovir alafenamide is considered as a complete regimen for the treatment of HIV-1 infection in treatment-naive patients or in patients virologically suppressed for at least 3 months with no history of treatment failure. Additionally, the combination product including [elvitegravir], [cobicistat], emtricitabine and tenofovir alafenamide and the combination product including emtricitabine, [rilpivirine] and tenofovir alafenamide can be used in the treatment of HIV-1 infection in patients older than 12 years with no previous antiretroviral therapy history or who are virologically suppressed for at least 6 months with no history of treatment failure. The combination product including [darunavir], cobicistat, emtricitabine, and tenofovir alafenamide is indicated for the treatment of HIV-1 infection in adults without prior antiretroviral therapy or in patients virologically suppressed for 6 months and no reported resistance to darunavir or tenofovir.
FDA Label
Vemlidy is indicated for the treatment of chronic hepatitis B in adults and adolescents (aged 12 years and older with body weight at least 35 kg).
Tenofovir alafenamide has been shown to be a potent inhibitor of hepatitis B viral replication. Tenofovir alafenamide presents a better renal tolerance when compared with the counterpart [tenofovir disoproxil]. This improved safety profile seems to be related to a lower plasma concentration of tenofovir. In clinical trials, tenofovir alafenamide was shown to present 5-fold more potent antiviral activity against HIV-1 when compared to tenofovir disoproxil.
J05AF
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
J05AF13 - Tenofovir alafenamide
Absorption
As compared to the parent molecule, [tenofovir], tenofovir alafenamide presents a lipophilic group that masks the negative charge of the parent moiety which improves its oral bioavailability. Tenofovir alafenamide is highly stable in plasma and, after administration of this prodrug, there is a low concentration of tenofovir in plasma. After oral administration, tenofovir alafenamide is rapidly absorbed by the gut. When a single dose is administered, a peak concentration of 16 ng/ml of the parent compound, corresponding to about 73% of the dose, is observed after 2 hours with an AUC of 270 ng\*h/mL. Once inside the body, tenofovir alafenamide enters hepatocytes by passive diffusion regulated by the organic anion transporters 1B1 and 1B3 for its activation. Administration of tenofovir alafenamide concomitantly with a high-fat meal results in an increase of about 65% in its internal exposure.
Route of Elimination
Tenofovir alafenamide has been registered to present a bile elimination that corresponds to 47% of the administered dose and a renal elimination the represents about 36%. From the recovered dose in urine, about 75% is represented as unchanged [tenofovir] followed by uric acid and a small dose of tenofovir alafenamide. On the other hand, in feces, 99% of the recovered dose corresponds to tenofovir.
Volume of Distribution
In clinical trials, the reported volume of distribution of tenofovir alafenamide was higher than 100 L.
Clearance
The reported clearance rate of tenofovir alafenamide is 117 L/h. In patients with severe renal impairment, this value can be decreased by 50%, reporting a rate of 61.7 L/h.
To be activated, tenofovir alafenamide is required to be hydrolyzed to the parent compound [tenofovir] by the activity of cathepsin A or carboxylesterase 1. Tenofovir alafenamide presents significant plasma stability and hence, its activation is performed inside the target cells. After activation, tenofovir is further processed and after 1-2 days, it is detected in plasma almost completely transformed to uric acid.
The reported half-life for tenofovir alafenamide is of 0.51 hours.
Tenofovir alafenamide presents 91% lower plasma concentration with an intracellular presence of about 20-fold higher when compared to [tenofovir disoproxil]. This is due to its prolonged systemic exposure and its higher intracellular accumulation of the active metabolite tenofovir diphosphate. Tenofovir alafenamide accumulates more in peripheral blood mononuclear cells compared to red blood cells. Once activated, tenofovir acts with different mechanisms including the inhibition of viral polymerase, causing chain termination and the inhibition of viral synthesis. To know more about the specific mechanism of action of the active form, please visit the drug entry of [tenofovir].