

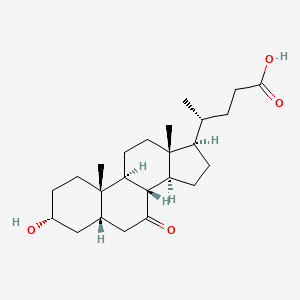

1. 3 Alpha-hydroxy-7-keto-5 Beta-cholanoate
2. 3 Alpha-ol-7-one-5 Beta-cholanoic Acid
3. 7-ketolithocholic Acid
4. 7-ketolithocholic Acid, (3beta,5alpha)-isomer
5. 7-oxolithocholic Acid
1. 4651-67-6
2. 7-ketolithocholic Acid
3. Nutriacholic Acid
4. 7-oxolithocholic Acid
5. 3a-hydroxy-7-oxo-5b-cholanic Acid
6. 3.alpha.-hydroxy-7-oxo-5.beta.-cholanic Acid
7. 3alpha-hydroxy-7-oxo-5beta-cholan-24-oic Acid
8. 3alpha-hydroxy-7-oxo-5beta-cholanoic Acid
9. Chebi:82679
10. Cholan-24-oic Acid, 3-hydroxy-7-oxo-, (3a,5b)-
11. Y1357h1a28
12. (r)-4-((3r,5s,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethyl-7-oxohexadecahydro-1h-cyclopenta[a]phenanthren-17-yl)pentanoic Acid
13. Cholan-24-oic Acid, 3-hydroxy-7-oxo-, (3.alpha.,5.beta.)-
14. 3alpha-hydroxy-7-keto-5beta-cholanic Acid
15. (4r)-4-[(3r,5s,8r,9s,10s,13r,14s,17r)-3-hydroxy-10,13-dimethyl-7-oxo-1,2,3,4,5,6,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]pentanoic Acid
16. (3alpha,5beta)-3-hydroxy-7-oxocholan-24-oic Acid
17. 7-ketochenodeoxycholic Acid
18. Ccris 3987
19. Mfcd00271393
20. Unii-y1357h1a28
21. Nsc-226118
22. Einecs 225-083-2
23. 3-alpha-hydroxy-7-oxo-5beta-cholanic Acid
24. 3-alpha-hydroxy-7-oxo-5-beta-cholanic Acid
25. Nsc 226118
26. 7-keto-lca
27. 7-keto-lithocholic Acid
28. Ec 225-083-2
29. Schembl234771
30. Chembl1254169
31. Amy1798
32. Dtxsid501032263
33. 3-hydroxy-7-oxo-5-cholanic Acid
34. 3?-hydroxy-7 Ketolithocholic Acid
35. Zinc3914814
36. Lmst04010150
37. Akos015855480
38. Akos016010910
39. Cs-w019298
40. Ds-4325
41. Hy-w018512
42. 3-hydroxy-7-oxocholan-24-oic Acid #
43. 7-oxo-3alpha-hydroxycholan-24-oic Acid
44. Ac-29218
45. 3alpha--hydroxy-7-oxo-5beta-cholanic Acid
46. 3i+/--hydroxy-7-oxo-5i(2)-cholanic Acid
47. H0869
48. F11720
49. 3alpha-hydroxy-7-keto-5beta-cholan-24-oic Acid
50. (3a,5ss)-3-hydroxy-7-oxo-cholan-24-?oic Acid
51. 7-oxo-3.alpha.-hydroxycholan-24-oic Acid
52. 3.alpha.-hydroxy-7-oxo-5.beta.-cholic Acid
53. Q27156201
54. Ursodeoxycholic Acid Impurity F [ep Impurity]
55. 3.alpha.-hydroxy-7-oxo-5.beta.-cholanoic Acid
56. Cholan-24-oic Acid, 3-hydroxy-7-oxo-, (3alpha,5beta)-
57. 3.alpha.-hydroxy-7-oxo-5.beta.-cholan-24-oic Acid
| Molecular Weight | 390.6 g/mol |
|---|---|
| Molecular Formula | C24H38O4 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 390.27700969 g/mol |
| Monoisotopic Mass | 390.27700969 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 645 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cholagogues and Choleretics
Gastrointestinal agents that stimulate the flow of bile into the duodenum (cholagogues) or stimulate the production of bile by the liver (choleretic). (See all compounds classified as Cholagogues and Choleretics.)