

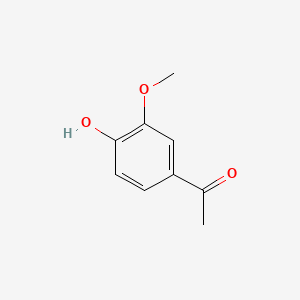

1. 4'-hydroxy-3'-methoxyacetophenone
2. 4-hydroxy-3-methoxyacetophenone
3. Apocynin
4. Apocynine
1. Apocynin
2. 498-02-2
3. 4'-hydroxy-3'-methoxyacetophenone
4. 1-(4-hydroxy-3-methoxyphenyl)ethanone
5. Acetoguaiacone
6. Apocynine
7. Acetoguaiacon
8. 4-acetyl-2-methoxyphenol
9. Acetovanilone
10. Acetovanyllon
11. 4-hydroxy-3-methoxyacetophenone
12. Ethanone, 1-(4-hydroxy-3-methoxyphenyl)-
13. 3-methoxy-4-hydroxyacetophenone
14. 1-(4-hydroxy-3-methoxyphenyl)ethan-1-one
15. 4-hydroxy-3-methoxyphenyl Methyl Ketone
16. Acetophenone, 4'-hydroxy-3'-methoxy-
17. Nsc 209524
18. 3-metoksy-4-hydroksyacetofenon
19. 2-methoxy-4-acetylphenol
20. Acetovanillon
21. B6j7b9udtr
22. Nsc-209524
23. Chebi:2781
24. 1-(4-hydroxy-3-methoxy-phenyl)-ethanone
25. Wln: 1vr Dq Co1
26. Einecs 207-854-5
27. Brn 0637373
28. Ccris 7285
29. Unii-b6j7b9udtr
30. 3-metoksy-4-hydroksyacetofenon [polish]
31. Ai3-15892
32. 4-acetylguaiacol
33. 1-(4-hydroxy-3-methoxy-phenyl)ethanone
34. Apocynin [mi]
35. Apocynin (acetovanillone)
36. Bmse000584
37. Bmse010031
38. Phenol, 4-acetyl-2-methoxy
39. 4-08-00-01814 (beilstein Handbook Reference)
40. Mls001304972
41. Schembl109514
42. 4-hydroxy3-methoxyacetophenone
43. 4hydroxy-3-methoxyacetophenone
44. Acetovanillone, >=98%, Fg
45. Chembl346919
46. Dtxsid7060097
47. 4-hydroxy -3-methoxyacetophenone
48. Nsc2146
49. Acetovanillone, Analytical Standard
50. Hms3651h03
51. Zinc162515
52. 3'-methoxy-4'-hydroxyacetophenone
53. Hy-n0088
54. Nsc 2146
55. Nsc-2146
56. Str03975
57. 3-methyl Methcathinone Hydrochloride
58. Bbl009710
59. Mfcd00008747
60. Nsc209524
61. S2425
62. Stl141075
63. Zinc00162515
64. Akos000120562
65. 4-hydroxy-3-methoxyphenyl Methyl Keton
66. Ccg-266327
67. Cs-5647
68. Db12618
69. Fs-3673
70. 1-(4-hydroxy-3-methoxyphenyl)-ethanone
71. Ncgc00247065-01
72. 4'-hydroxy-3'-methoxyacetophenone, 98%
73. Ac-29981
74. Smr000752909
75. 1-(4-hydroxy-3-methoxyphenyl)-1-ethanone
76. Am20090774
77. Ft-0618638
78. H0261
79. Sw219526-1
80. D70564
81. Aa-504/20839006
82. Q414754
83. Q-200477
84. Acetophenone,4-hydroxy,3-methoxy Acetovanillon
85. F2191-0004
86. 16522-48-8
87. I75
| Molecular Weight | 166.17 g/mol |
|---|---|
| Molecular Formula | C9H10O3 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 166.062994177 g/mol |
| Monoisotopic Mass | 166.062994177 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 167 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)