


1. Centrophne
2. Dihydrochloride, Trimetazidine
3. Idaptan
4. Trimtazidine Irex
5. Trimetazidine Dihydrochloride
6. Vasartel
7. Vastarel
1. 5011-34-7
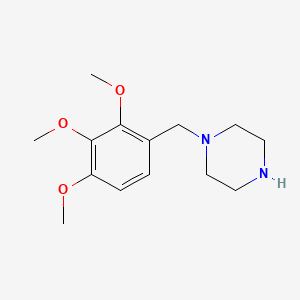
2. 1-(2,3,4-trimethoxybenzyl)piperazine
3. 1-[(2,3,4-trimethoxyphenyl)methyl]piperazine
4. 1-(2,3,4-trimethoxy-benzyl)-piperazine
5. Piperazine, 1-((2,3,4-trimethoxyphenyl)methyl)-
6. N9a0a0r9s8
7. Trimetazidine (inn)
8. Trimetazidine [inn]
9. Piperazine, 1-[(2,3,4-trimethoxyphenyl)methyl]-
10. Trimetazidina
11. Trimetazidinum
12. Mls001240268
13. Trimetazidinum [inn-latin]
14. Trimetazidina [inn-spanish]
15. Trimetazidine [inn:ban:dcf]
16. Ncgc00016697-01
17. Smr000674573
18. Einecs 225-690-2
19. Cas-13171-25-0
20. Unii-n9a0a0r9s8
21. Preductal
22. Vasorel
23. Preductal Mb
24. Dilatan (tn)
25. Bas 06612844
26. Prestwick0_000549
27. Prestwick1_000549
28. Prestwick2_000549
29. Prestwick3_000549
30. Trimetazidine [mi]
31. Oprea1_279550
32. Piperazine,1-[(2,3,4-trimethoxyphenyl)methyl]-
33. Bspbio_000597
34. Mls001331735
35. Schembl230374
36. Spbio_002518
37. Trimetazidine [who-dd]
38. Bpbio1_000657
39. Chembl203266
40. Dtxsid2048531
41. Bdbm80613
42. Chebi:94789
43. Cid_9926449
44. Hy-b0968a
45. Hms2230l07
46. Hms3374d04
47. Albb-004703
48. Bcp16534
49. Bbl013084
50. Mfcd00868263
51. S5779
52. Stk315643
53. Zinc19358638
54. Akos000308094
55. 4-(2,3,4-trimethoxybenzyl)piperazine
56. Db09069
57. Sb75326
58. 1-(2,3,4-trimethoxy Benzyl)piperazine
59. Ncgc00016697-02
60. Smr000814701
61. 1-(2,3,4-trimethoxyphenyl)methylpiperazine
62. 1-(2,3,4-trimethoxyphenylmethyl)piperazine
63. Db-051731
64. Bb 0220635
65. Cs-0099250
66. En300-14439
67. 71t250
68. D08642
69. A827982
70. A837947
71. Q674703
72. 1-(2,3,4-trimethoxybenzyl)piperazine;hydrochloride
73. 1-[2,3,4-trimethoxybenzyl] Piperazine Dihydrochloride
74. Brd-k88366685-300-03-7
75. Brd-k88366685-300-04-5
76. Z99601262
77. 1-[(2,3,4-trimethoxyphenyl)methyl]piperazine;hydrochloride
78. 127881-54-3
| Molecular Weight | 266.34 g/mol |
|---|---|
| Molecular Formula | C14H22N2O3 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 266.16304257 g/mol |
| Monoisotopic Mass | 266.16304257 g/mol |
| Topological Polar Surface Area | 43 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 259 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Trimetazidine is indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies.
Trimetazidine is indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies. Patients should be counselled regarding the risk of use with reduced renal or hepatic function, worsening of extrapyramidal symptoms or other movement disorders, and risk of falls.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C01EB15
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01E - Other cardiac preparations
C01EB - Other cardiac preparations
C01EB15 - Trimetazidine
Absorption
In elderly patients, a 35 mg oral modified release tablet reaches a mean Cmax of 115 g/L, with a Tmax of 2.0-5.0 hours, and a mean AUC0-12 of 1104 h\*g/L. In young, healthy patients, the same dose reaches a mean Cmax of 91.2 g/L, with a Tmax of 2.0-6.0 hours, and an AUC0-12h 720 h\*g/L.
Route of Elimination
Trimetazidine is 79-84% eliminated in the urine, with 60% as the unchanged parent compound. In a study of 4 healthy subjects, individual metabolites made up 0.01-1.4% of the dose recovered in urine. In the urine, 2-desmethyltrimetazidine made up 0-1.4% of the recovered dose, 3- and 4-desmethyltrimetazidine made up 0.039-0.071% each, N-methyltrimetazidine made up 0.015-0.11%, trimetazidine ketopiperazine made up 0.011-0.4%, N-formyltrimetazidine made up 0.035-0.42%, N-acetyltrimetazidine made up 0.016-0.19%, desmethyl trimetazidine O-sulphate made up 0.01-0.65%, and an unknown metabolite made up0.026-0.67%.
Volume of Distribution
The volume of distribution of trimetazidine is 4.8 L/kg.
Clearance
Trimetazidine clearance is strongly correlated with creatinine clearance. In eldery patients with a creatinine clearance of 72 8 mL/min, trimetazidine clearance was 15.69 L/h. In young, healthy patients with a creatinine clearance of 134 18 mL/min, trimetazidine clearance was 25.2 L/h.
Trimetazidine can be oxidized at the piperazine ring to form trimetazidine ketopiperazine. Trimetazidine can also be N-formylated, N-acetylated, or N-methylated at the piperazine ring to form N-formyltrimetazidine, N-acetyltrimetazidine, and N-methyltrimetazidine respectively. Alternatively, trimetazidine can be demethylated at the 2, 3, or 4 position of the 2,3,4-trimethoxybenzyl moiety to form 2-desmethyltrimetazidine, 3-desmethyltrimetazidine, or 4-desmethyltrimetazidine. The desmethyltrimetazidine metabolites can undergo sulfate conjugation or glucuronidation prior to elimination.
In young, healthy subjects, the half life of trimetazidine is 7.81 hours. In patients over 65, the half life increases to 11.7 hours.
During myocardial ischemia, anaerobic metabolism takes over, increasing levels of lactic acid. The decreased intracellular pH and increased concentration of protons activates sodium-hydrogen and sodium-calcium antiport systems, raising intracellular calcium concentrations, finally leading to decreased contractility. This injury to the myocardium raises concentrations of catecholamines, which activate hormone sensitive lipase, and increasing fatty acid concentrations in plasma. When the myocardium is repurfused, fatty acid oxidation becomes the dominant form of ATP production, maintaining an acidic pH, and further exacerbating the injury. The mechanism of action of trimetazidine is not fully understood. Trimetazidine may inhibit mitochondrial 3-ketoacyl coenzyme A thiolase, decreasing long chain fatty acid -oxidation but not glycolysis in the myocardium. The decreased long chain fatty acid -oxidation is compensated for by increased use of glucose, preventing a lowered myocardial pH, and further decreases in contractility. However, another study suggests that 3-ketoacyl coenzyme A thiolase may not be trimetazidine's target, and that this mechanism may be incorrect.