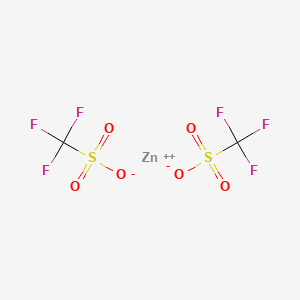

1. Zn(otf)(2)
2. Zn(otf)2
1. 54010-75-2
2. Zinc(ii) Trifluoromethanesulfonate
3. Zinc Trifluoromethanesulphonate
4. Zinc Triflate
5. Zinc Trifluoromethansulfonate
6. Zinc;trifluoromethanesulfonate
7. Methanesulfonic Acid, Trifluoro-, Zinc Salt
8. Zn(otf)2
9. Zinc(ii) Triflate
10. Zinktriflat
11. Zinc(ii)triflate
12. Einecs 258-922-6
13. Mfcd00013229
14. Zinc (ii) Triflate
15. Schembl165265
16. Zinc Trifluorometha-nesulfonate
17. Dtxsid7068899
18. Zinc(ii)trifluoromethanesulfonate
19. Methanesulfonic Acid, 1,1,1-trifluoro-, Zinc Salt (2:1)
20. Zinc (ii) Trifluoromethanesulfonate
21. Act09824
22. Amy37817
23. Zinc Trifluoromethanesulfonate, 98%
24. Akos005258662
25. Gs-6843
26. Sc10875
27. Zinc(2+) Ion Ditrifluoromethanesulfonate
28. Zinc(ii) Trifluoromethanesulphonate
29. Db-009916
30. Trifluoromethanesulfonic Acid Zinc(ii) Salt
31. Ft-0658247
32. T1294
33. A829906
34. Q8072312
35. Trifluoromethanesulfonic Acid Zinc Salt, Zinc Triflate
| Molecular Weight | 363.5 g/mol |
|---|---|
| Molecular Formula | C2F6O6S2Zn |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 131 |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 145 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Zinc can enter the body through the lungs, skin, and gastrointestinal tract. Intestinal absorption of zinc is controlled by zinc carrier protein CRIP. Zinc also binds to metallothioneins, which help prevent absorption of excess zinc. Zinc is widely distributed and found in all tissues and tissues fluids, concentrating in the liver, gastrointestinal tract, kidney, skin, lung, brain, heart, and pancreas. In the bloodstream zinc is found bound to carbonic anhydrase in erythrocytes, as well as bound to albumin, _2-macroglobulin, and amino acids in the the plasma. Albumin and amino acid bound zinc can diffuse across tissue membranes. Zinc is excreted in the urine and faeces. (L49)