

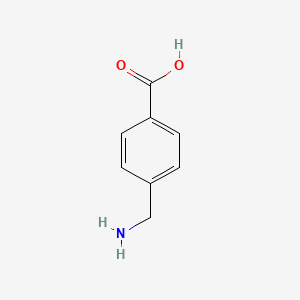

1. 4-aminomethylbenzoic Acid
2. P-aminomethylbenzoic Acid
3. Pamba
4. Para-aminomethylbenzoic Acid
1. 56-91-7
2. 4-carboxybenzylamine
3. Aminomethylbenzoic Acid
4. 4-aminomethylbenzoic Acid
5. Pamba
6. Styptopur
7. P-aminomethylbenzoic Acid
8. Gumbix
9. Benzoic Acid, 4-(aminomethyl)-
10. Benzylamine-4-carboxylic Acid
11. Alpha-amino-p-toluic Acid
12. P-(aminomethyl)benzoic Acid
13. 4-aminomethyl-benzoic Acid
14. Mfcd00010203
15. Nsc41629
16. Nsc-41629
17. 68wg9jkc7l
18. .alpha.-amino-p-toluic Acid
19. Chembl328875
20. P-toluic Acid, .alpha.-amino-
21. 4-(aminomethyl)benzoesaeure
22. Einecs 200-297-9
23. Nsc 41629
24. Unii-68wg9jkc7l
25. 4-(aminomethyl)benzoicacid
26. P-toluic Acid, Alpha-amino-
27. Gumbix (tn)
28. Pteroicacid
29. A-amino-p-toluic Acid
30. 4-aminomethylbezoic Acid
31. Alphaamino-p-toluic Acid
32. Tranexamic Acid Impurity D
33. 4-aminomethyl Benzoic Acid
34. 4-(aminomethyl)benzoic-acid
35. Ncistruc1_000124
36. Ncistruc2_000164
37. Oprea1_689394
38. Schembl38758
39. Cbdive_002627
40. 4-(aminomethyl) Benzoic Acid
41. 4-(aminomethyl)-benzoic Acid
42. Gtpl4702
43. Dtxsid20204568
44. Chebi:134774
45. Act00964
46. Bcp24445
47. Hy-b1258
48. Nci41629
49. 4-(aminomethyl)benzoic Acid, 97%
50. 4-(aminomethyl)phenylcarboxylic Acid
51. Bdbm50408790
52. Ccg-36812
53. Ncgc00013487
54. Stk246898
55. Zinc12359009
56. Akos000118982
57. Aminomethylbenzoic Acid [mart.]
58. Cs-4892
59. Db13244
60. Aminomethylbenzoic Acid [who-dd]
61. Ncgc00013487-02
62. Ncgc00096601-01
63. Ac-10932
64. As-13170
65. Nci60_003944
66. Sy002868
67. Tranexamic Acid Related Compound D
68. Db-008385
69. A0965
70. Am20060672
71. Ft-0616689
72. D07568
73. D70590
74. Tranexamic Acid Impurity D [ep Impurity]
75. Q695442
76. W-105499
77. Aminomethylbenzoic Acid 4-(aminomethyl)benzoic Acid
78. F1313-0009
79. Tranexamic Acid Related Compound D [usp Impurity]
80. 4az
| Molecular Weight | 151.16 g/mol |
|---|---|
| Molecular Formula | C8H9NO2 |
| XLogP3 | -1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 151.063328530 g/mol |
| Monoisotopic Mass | 151.063328530 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 139 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antifibrinolytic Agents
Agents that prevent fibrinolysis or lysis of a blood clot or thrombus. Several endogenous antiplasmins are known. The drugs are used to control massive hemorrhage and in other coagulation disorders. (See all compounds classified as Antifibrinolytic Agents.)
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
B - Blood and blood forming organs
B02 - Antihemorrhagics
B02A - Antifibrinolytics
B02AA - Amino acids
B02AA03 - Aminomethylbenzoic acid