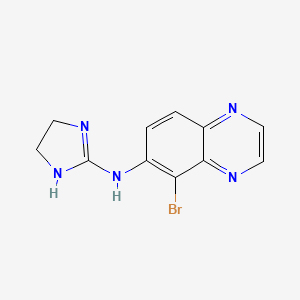

1. 5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline D-tartrate
2. 5-bromo-6-(imidazolidinylideneamino)quinoxaline
3. 5-bromo-6-(imidazolin-2-ylamino)quinoxaline
4. Agn 190342
5. Agn-190342
6. Agn190342
7. Alphagan
8. Alphagan P
9. Brimonidine Purite
10. Brimonidine Tartrate
11. Brimonidine Tartrate (1:1)
12. Brimonidine Tartrate (1:1), (s-(r*,r*))-isomer
13. Brimonidine Tartrate, (r-(r*,r*))-isomer
14. Bromoxidine
15. Mirvaso
16. Ratio Brimonidine
17. Ratio-brimonidine
18. Sanrosa
19. Uk 14,304
20. Uk 14,304 18
21. Uk 14,304-18
22. Uk 14,30418
23. Uk 14,308
24. Uk 14304
25. Uk 14308
26. Uk-14,304-18
27. Uk-14,308
28. Uk-14304
29. Uk14,30418
30. Uk14,308
31. Uk14304
1. 59803-98-4
2. Bromoxidine
3. Uk 14,304
4. 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)quinoxalin-6-amine
5. 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)-6-quinoxalinamine
6. 5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline
7. Uk-14304
8. 5-bromo-6-(imidazolin-2-ylamino)quinoxaline
9. Agn 190342
10. 6-quinoxalinamine, 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)-
11. Uk 14304
12. Agn-190342
13. Mls000069370
14. E6gnx3hhte
15. Mfcd00153878
16. Chembl844
17. Nsc-318825
18. Smr000058355
19. Chebi:3175
20. Agn-190342 Free Base
21. Brimonidine (inn)
22. Uk-1430418 Free Base
23. Ncgc00016069-09
24. Uk 14304;agn190342
25. Brimonidine [inn]
26. Dsstox_cid_25221
27. Dsstox_rid_80758
28. Dsstox_gsid_45221
29. Uk 14304 (tartrate);agn190342 (tartrate)
30. Brimonidinum
31. Brimonidine [inn:ban]
32. [3h]brimonidine
33. Cas-59803-98-4
34. N-(5-bromoquinoxalin-6-yl)imidazolidin-2-imine
35. Sr-01000000023
36. Unii-e6gnx3hhte
37. Brimonidina
38. Brn 0751629
39. Lk 14304-18
40. Agn190342
41. Uk14304
42. Tocris-0425
43. [3h]-uk14304
44. Opera_id_612
45. Lopac-u-104
46. Brimonidine [mi]
47. Brimonidine [vandf]
48. Cid_2435
49. Lopac0_001216
50. Schembl24670
51. Brimonidine [who-dd]
52. Gtpl520
53. 5-bromo-n-(2-imidazolin-2-yl)-6-quinoxalinamine
54. Mls001076349
55. Bidd:gt0649
56. Gtpl5386
57. Dtxsid3045221
58. Bdbm34572
59. 5-bromo-n-(4,5-dihydro-2-imidazolyl)quinoxalin-6-amine
60. Hms3259p09
61. Hms3263d14
62. Hms3266o03
63. Hms3411k05
64. Hms3675k05
65. Hms3887k07
66. Amy22318
67. Bcp12632
68. Ex-a5415
69. Hy-b0659
70. Tox21_110299
71. Tox21_501216
72. Ac-162
73. Nsc318825
74. Pdsp1_000640
75. Pdsp2_000635
76. S9508
77. Zinc21303210
78. Akos005267239
79. Tox21_110299_1
80. Ccg-205290
81. Db00484
82. Gs-3236
83. Lp01216
84. Nc00638
85. Nsc 318825
86. Sdccgsbi-0051183.p002
87. Mrf-0000657
88. Ncgc00016069-01
89. Ncgc00016069-02
90. Ncgc00016069-03
91. Ncgc00016069-04
92. Ncgc00016069-05
93. Ncgc00016069-06
94. Ncgc00016069-07
95. Ncgc00016069-08
96. Ncgc00016069-10
97. Ncgc00016069-11
98. Ncgc00016069-12
99. Ncgc00016069-13
100. Ncgc00016069-24
101. Ncgc00023468-02
102. Ncgc00023468-04
103. Ncgc00023468-05
104. Ncgc00023468-06
105. Ncgc00023468-07
106. Ncgc00261901-01
107. Sy053060
108. Uk14,304
109. Brimonidine 100 Microg/ml In Acetonitrile
110. B4132
111. Eu-0101216
112. Ft-0630717
113. Ft-0650586
114. En300-50880
115. Uk 14304-18
116. C07886
117. C75796
118. D07540
119. 5-bromo-6-(2-imidazolidinylidenamino)quinoxaline
120. 5-bromo-6-(2-imidazolin-2-ylamino) Quinoxaline
121. 5-bromo-6-(2-imidazolin-2-ylamino)-quinoxaline
122. 6-quinoxalinamine,5-dihydro-1h-imidazol-2-yl)-
123. 803b984
124. A832477
125. L000615
126. Q577377
127. Sr-01000000023-2
128. Sr-01000000023-4
129. Brd-k68264559-001-10-0
130. Z802671510
131. (5-bromo-quinoxalin-6-yl)-(4,5-dihydro-1h-imidazol-2-yl)-amine
132. 5-bromanyl-n-(4,5-dihydro-1h-imidazol-2-yl)quinoxalin-6-amine
133. 5-bromo-6-(2-imidazolin-2-ylamino)quinoxaline D-tartrate (1:1).
134. 6-quinoxalinamine, 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)- (9ci)
135. 6-quinoxalinamine, 5-bromo-n-(4,5-dihydro-1h-imidazol-2-yl)-, (s-(r*,r*))-2,3-dihydroxybutanedioate (1:1)
| Molecular Weight | 292.13 g/mol |
|---|---|
| Molecular Formula | C11H10BrN5 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 291.01196 g/mol |
| Monoisotopic Mass | 291.01196 g/mol |
| Topological Polar Surface Area | 62.2 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 308 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
**Opthalmic** Indicated for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension as monotherapy or combination product with [brinzolamide]. **Topical** Indicated for the treatment of persistent (non-transient) facial erythema of rosacea in adults 18 years of age or older.
FDA Label
Treatment of conjunctival hyperaemia
Brimonidine is a highly selective alpha-2 adrenergic receptor agonist that is 1000-fold more selective for the alpha2-adrenergic receptor than the alpha1-adrenergic receptor. This characteristic gives the drug some therapeutic advantages, since it reduces the risk of systemic side effects, such as systemic hypotension, bradycardia, and sedation. In addition, there is a reduction in the risk for developing alpha-1 mediated ocular unwanted effects, such as conjunctival blanching, mydriasis, and eyelid retraction. However, despite high alpha-2 receptor specificity, brimonidine may still produce alpha-1 adrenoceptor-mediated ocular effects, such as conjunctival vasoconstriction. Brimonidine has a peak ocular hypotensive effect occurring at two hours post-dosing. In a randomized, double-blind clinical study, ocular administration of 0.2% brimonidine in healthy volunteers resulted in a 23% reduction of mean intraocular pressure from baseline at 3 hours following administration. In comparative studies consisting of patients with open-angle glaucoma or ocular hypertension, the ocular hypotensive effect of brimonidine was maintained during treatment periods of up to 1 year. Brimonidine mediates vasoconstrictive effects and it was shown to exhibit anti-inflammatory properties in _ex vivo_ human skin model and _in vivo_ inflammation models. In a clinial trials consisting of adults with moderate to severe facial erythema of rosacea, brimonidine was shown to improve the extent of redness at 3 hours after application, compared to placebo. It was shown to be a potent vasoconstrictor of human subcutaneous vessels with a diameter of less than 200 m. In _in vivo_ mouse inflammation models, brimonidine displayed anti-inflammatory properties by inhibiting edema. In a randomized, double-blind study, brimonidine reduced erythema for the 12 hours of the study in a dose-dependent manner. When adminsitered systemically, brimonidine was shown to cause cardiovascular effects by decreasing blood pressure, decreasing heart and respiratory rate, and prolonging the PR interval in the electrocardiogram. This is due to the targeting of adrenoceptors by the drug. Although the clinical significance has not been established, there is evidence that brimonidine exhibits neuroprotective activity in experimental models of cerebral ischemia and optic nerve injury. _In vitro_ studies show that brimonidine mediated protective effects on neuronal cells from kainate acid insult and on cultured retinal ganglion cells from glutamate-induced cytotoxicity, which is a possible mediator of secondary neuronal degeneration in human glaucoma. Neuroprotective actions of brimonidine were also demonstrated in rat models of acute retinal ischemia and chronic IOP elevation. It has been proposed that brimonidine may exert neuroprotective effects on the retina and optic nerve by enhancing intrinsic retinal ganglion cell survival mechanisms and/or induction of neuronal survival factors, such as bFGF. However, further investigations are needed to conclude on these possible therapeutic benefits of the drug.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Adrenergic alpha-2 Receptor Agonists
Compounds that bind to and activate ADRENERGIC ALPHA-2 RECEPTORS. (See all compounds classified as Adrenergic alpha-2 Receptor Agonists.)
S01EA05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX21 - Brimonidine
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EA - Sympathomimetics in glaucoma therapy
S01EA05 - Brimonidine
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GA - Sympathomimetics used as decongestants
S01GA07 - Brimonidine
Absorption
Brimonidine readily penetrates the cornea following ocular administration to reach pharmacologically active concentrations in the aqueous humor and ciliary body, the putative sites of its IOP-lowering activity. Following ocular administration of 0.2% brimonidine solution, the peak plasma concentrations were achieved within 1 to 4 hours. In a clinical study of adult subjects with facial erythema of rosacea, brimonidine was cutaneously applied on facial skin in a repeated manner. While there was no drug accumulation in plasma, the highest peak plasma concentrations (Cmax) and AUC were 46 62 pg/mL and 417 264 pgxhr/mL, respectively.
Route of Elimination
Brimonidine and its metabolites are predominantly eliminated via urinary excretion, with 74% of the total dose being found in the urine.
Volume of Distribution
The volume of distribution of brimonidine has not been established. In animal studies, brimonidine was shown to cross the placenta and enter into the fetal circulation to a limited extent. As its lipophilicity is relatively low, brimonidine is not reported to easily cross the blood-brain barrier.
Clearance
The apparent clearance has not been studied. However, the systemic clearance of brimonidine is reported to be rapid. Approximately 87% of the total radioactive dose of brimonidine was shown to be eliminated within 120 hours following oral administration.
Brimonidine is reported to be metabolized in the cornea. Brominidine that reaches the systemic circulation upon topical administration undergoes extensive hepatic metabolism mediated by hepatic aldehyde oxidases.
Following ocular administration of 0.2% brimonidine solution, the systemic half-life was approximately 3 hours.
In the eye, alpha-1 adrenoceptors play a role in vasoconstriction, mydriasis, eyelid retraction, and elevation of intraocular pressure (IOP) whereas alpha-2 adrenoceptors are responsible for IOP reduction via a complex Gi-coupled signaling cascade pathway. Activation of alpha-2 receptors leads to inhibition of adenylyl cyclase and reduction of cyclic AMP levels. As a result, there is a decrease in norpinephrine (NE) release at the synaptic junction, NE-induced stimulation of beta-2 adrenoceptors, and production of aqueous humor by the ciliary epithelium. An elevated IOP is the most significant risk factor for developing glaucomatous optic neuropathy, which is associated with progressive visual field loss and functional disability if left untreated. Regardless of the etiology of the disease, the aim of current therapies for glaucoma is to reduce IOP, as reduction of IOP significantly reduces the risk of progression of vision loss even when IOP is already within the normal range. When administered ophthalmically, brimonidine is rapidly absorbed into the eye, acts as an agonist at ocular alpha-2 adrenoceptors and lowers IOP via a dual mechanism of action. It is proposed that initial dosing of the drug causes a reduction in aqueous humour production and chronic dosing leads to an increase in uveoscleral outflow. Brimonidine does not affect episcleral venous pressure. By reducing IOP, brimonidine aims to reduce the likelihood of glaucomatous visual field loss in ocular hypertension, and slow the progression of visual field defect in established open-angle glaucoma. When applied topically on skin, brimonidine reduces erythema through direct vasocontriction of small arteries and veins. As brimonidine mediates a potent peripheral vasoconstrictive activity by selectively working on the alpha-2 adrenoceptors, the use of brimonidine is thought to be efficacious for the treatment of facial erythema of rosacea, which is thought to arise from vasomotor instability and abnormal vasodilation of the superficial cutaneous vasculature of the face.