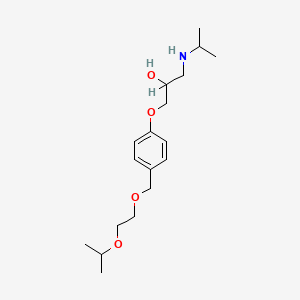

1. Bisoprolol Fumarate
2. Bisoprolol Fumarate (1:1) Salt, (+-)-isomer
3. Bisoprolol Fumarate (2:1) Salt, (+-)-isomer
4. Bisoprolol Hydrochloride
5. Bisoprolol Methanesulfonate Salt
6. Bisoprolol, (+-)-isomer
7. Bisoprolol, (-)-isomer
8. Bisoprolol, Fumarate (1:1) Salt
9. Bisoprolol, Fumarate (2:1) Salt
10. Cl 297939
11. Cl-297939
12. Cl297939
13. Concor
14. Emd 33512
15. Emd-33512
16. Emd33512
17. Fumarate, Bisoprolol
18. Hydrochloride, Bisoprolol
1. 66722-44-9
2. Bisoprololum
3. Bisoprolol Fumarate
4. Concor
5. Zebeta
6. Bisoprolol Hemifumarate
7. 1-(propan-2-ylamino)-3-[4-(2-propan-2-yloxyethoxymethyl)phenoxy]propan-2-ol
8. Emd-33512
9. Emd 33 512
10. Cl-297939
11. (rs)-1-(4-(2-isopropoxyethoxymethyl)phenoxy)-3-(isopropylamino)-2-propanol
12. (+-)-1-((alpha-(2-isopropoxyethoxy)-p-tolyl)oxy)-3-(isopropylamino)-2-propanol
13. Y41js2nl6u
14. Chebi:3127
15. Bisoprolol Hemifumarate Salt
16. (+/-)-bisoprolol (hemifumarate)
17. Bisocor
18. Bisoprololum [latin]
19. 1-(4-((2-isopropoxyethoxy)methyl)phenoxy)-3-(isopropylamino)propan-2-ol
20. [2-hydroxy-3-(4-{[2-(propan-2-yloxy)ethoxy]methyl}phenoxy)propyl](propan-2-yl)amine
21. 2-propanol, 1-[4-[[2-(1-methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-
22. Bisoprolol Fumerate
23. 1-{4-[(2-isopropoxyethoxy)methyl]phenoxy}-3-(isopropylamino)propan-2-ol
24. 1-(propan-2-ylamino)-3-(4-{[2-(propan-2-yloxy)ethoxy]methyl}phenoxy)propan-2-ol
25. Ncgc00024868-03
26. 104344-23-2
27. Unii-y41js2nl6u
28. Bisoprolol [usan:inn:ban]
29. Cl 297939
30. Sr-01000597980
31. Bisoprolol [mi]
32. Bisoprolol [inn]
33. Bisoprolol [jan]
34. Prestwick0_000330
35. Prestwick1_000330
36. Prestwick2_000330
37. Prestwick3_000330
38. Bisoprolol [usan]
39. Bisoprolol [vandf]
40. Chembl645
41. Bisoprolol [who-dd]
42. Schembl20960
43. Bspbio_000339
44. Bisoprolol (jan/usan/inn)
45. Spbio_002260
46. Bisoprolol, Analytical Standard
47. Bpbio1_000373
48. Gtpl7129
49. Dtxsid6022682
50. Bdbm25751
51. Hsdb 8316
52. Bcpp000332
53. Bcp02461
54. Ex-a5544
55. Mfcd00865795
56. S5942
57. Stl355996
58. Akos005110983
59. Bcp9000418
60. Db00612
61. Sdccgsbi-0206894.p002
62. Ncgc00024868-02
63. Ncgc00024868-04
64. Ncgc00024868-05
65. Ncgc00024868-06
66. Ncgc00024868-08
67. Ncgc00024868-21
68. 1-[(1-methylethyl)amino]-3-({4-[({2-[(1-methylethyl)oxy]ethyl}oxy)methyl]phenyl}oxy)propan-2-ol
69. 2-propanol, 1-(4-((2-(1-methylethoxy)ethoxy)methyl)phenoxy)-3-((1-methylethyl)amino)-, (+-)-
70. Ac-18540
71. As-35262
72. Sbi-0206894.p001
73. Hy-129029
74. Ab00514681
75. Bb 0262613
76. Cs-0103208
77. Bisoprolol - From Tablet Donated By Watson -
78. C06852
79. D02342
80. Ab00514681-08
81. Ab00514681_09
82. Ab00514681_10
83. 722b449
84. L000133
85. Q412515
86. ((c)i)-bisoprolol-d7 Hemifumarate (iso-propyl-d7)
87. Q-200728
88. Sr-01000597980-1
89. Brd-a89175223-051-05-6
90. 1-(4-[(2-isopropoxyethoxy)methyl]phenoxy)-3-(isopropylamino)-2-propanol #
91. 1-[isopropylamino]-3-[isopropoxyethoxymethylphenoxy]-2-propanol
92. (+/-)-1-((.alpha.-(2-isopropoxyethoxy)-p-tolyl)oxy)-3-(isopropylamino)-2-propanol
93. (.+/-.)-1-[[.alpha.-(2-isopropoxyethoxy)-p-tolyl]oxy]-3-(isopropylamino)-2-propanol
94. 1-(propan-2-ylamino)-3-[4-(2-propan-2-yloxyethoxymethyl)phenoxy]propan- 2-ol
95. 1-[4-[2-(1-methylethoxy)ethoxymethyl]phenoxy]-3-(1-methylethylamino)propan-2-ol
96. 1-{4-{[2-(1-methylethoxy)ethoxy]methyl}phenoxy}-3-[(1-methylethyl)amino]-2-propanol
97. 2-propanol, 1-(4-((2-(1-methylethoxy)ethoxy)methyl)phenoxy)-3-((1-methylethyl)amino)-, (+/-)-
98. 2-propanol, 1-[4-[[2-(1-methylethoxy)ethoxy]methyl]phenoxy]-3-[(1-methylethyl)amino]-, (.+/-.)-
| Molecular Weight | 325.4 g/mol |
|---|---|
| Molecular Formula | C18H31NO4 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 12 |
| Exact Mass | 325.22530847 g/mol |
| Monoisotopic Mass | 325.22530847 g/mol |
| Topological Polar Surface Area | 60 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 278 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic beta-1 Receptor Antagonists; Antihypertensive Agents; Sympatholytics
National Library of Medicine's Medical Subject Headings. Bisoprolol. Online file (MeSH, 2016). Available from, as of January 20, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Bisoprolol is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=bisoprolol&Search=Search
Zebeta is indicated in the management of hypertension. It may be used alone or in combination with other antihypertensive agents. /Included in US product label/
NIH; DailyMed. Current Medication Information for Zebeta (Bisoprolol Fumarate) Tablet, Film Coated (Updated: January 2016). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a11548a0-9c0f-4729-907c-75d8f99a6c85
MEDICATION (VET): Bisoprolol is a beta1 blocker that is somewhat cardioselective and therefore is indicated for conditions that require a reduction in heart rate, heart conductivity, or contractility. Such conditions include tachyarrhythmias and atrial fibrillation. In people it is used to treat hypertension, but this use has not been explored in animals.
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 74
For more Therapeutic Uses (Complete) data for Bisoprolol (6 total), please visit the HSDB record page.
Zebeta is contraindicated in patients with cardiogenic shock, overt cardiac failure, second or third degree AV block, and marked sinus bradycardia.
NIH; DailyMed. Current Medication Information for Zebeta (Bisoprolol Fumarate) Tablet, Film Coated (Updated: January 2016). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a11548a0-9c0f-4729-907c-75d8f99a6c85
VET: Use cautiously in animals with airway disease, myocardial failure, and cardiac conduction disturbances. Use cautiously in animals with low cardiac reserve.
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 75
Special caution should be exercised when administering bisoprolol fumarate to patients with a history of severe heart failure. Safety and effectiveness of bisoprolol doses higher than 10 mg/day in patients with heart failure have not been established. Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure and inhibition with beta-blockade always carries the potential hazard of further depressing myocardial contractility and precipitating cardiac failure. In general beta-blocking agents should be avoided in patients with overt congestive heart failure. However, in some patients with compensated cardiac failure, it may be necessary to utilize them. In such a situation, they must be used cautiously. Bisoprolol fumarate acts selectively without abolishing the effects of digitalis. However, the positive inotropic effect of digitalis may be reduced by the negative inotropic effect of bisoprolol fumarate when the two drugs are used concomitantly. The effects of beta-blockers and digitalis are additive in depressing A-V conduction.
Health Canada; Product Monograph for Bisoprolol (Bisoprolol Fumarate Tablets), Drug Identification Number (DIN): 02383055 p.4 (Date of Approval: April 5, 2012). Available from, as of March 11, 2016: https://webprod5.hc-sc.gc.ca/dpd-bdpp/start-debuter.do?lang=eng
Exacerbation of angina pectoris, and, in some instances, myocardial infarction or ventricular arrhythmia, have been observed in patients with coronary artery disease following abrupt cessation of therapy with beta-blockers. Such patients should, therefore, be cautioned against interruption or discontinuation of therapy without the physician's advice. Even in patients without overt coronary artery disease, it may be advisable to taper therapy with Zebeta over approximately one week with the patient under careful observation. If withdrawal symptoms occur, Zebeta therapy should be reinstituted, at least temporarily.
NIH; DailyMed. Current Medication Information for Zebeta (Bisoprolol Fumarate) Tablet, Film Coated (Updated: January 2016). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a11548a0-9c0f-4729-907c-75d8f99a6c85
For more Drug Warnings (Complete) data for Bisoprolol (17 total), please visit the HSDB record page.
Bisoprolol is indicated for the treatment of mild to moderate hypertension. It may be used off-label to treat heart failure, atrial fibrillation, and angina pectoris.
Bisoprolol decreases heart rate (chronotropy), decreases contractility (inotropy), and reduces blood pressure. The results of various clinical studies indicate that bisoprolol reduces cardiovascular mortality and all-cause mortality in patients with heart failure and decreased cardiac ejection fraction (EF).
Adrenergic beta-1 Receptor Antagonists
Drugs that bind to and block the activation of ADRENERGIC BETA-1 RECEPTORS. (See all compounds classified as Adrenergic beta-1 Receptor Antagonists.)
Sympatholytics
Drugs that inhibit the actions of the sympathetic nervous system by any mechanism. The most common of these are the ADRENERGIC ANTAGONISTS and drugs that deplete norepinephrine or reduce the release of transmitters from adrenergic postganglionic terminals (see ADRENERGIC AGENTS). Drugs that act in the central nervous system to reduce sympathetic activity (e.g., centrally acting alpha-2 adrenergic agonists, see ADRENERGIC ALPHA-AGONISTS) are included here. (See all compounds classified as Sympatholytics.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C07AB07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C07 - Beta blocking agents
C07A - Beta blocking agents
C07AB - Beta blocking agents, selective
C07AB07 - Bisoprolol
Absorption
Bisoprolol is well absorbed in the gastrointestinal tract. The AUC is 642.87 g.hr/mL and bioavailability of bisoprolol is about 90% due to the minimal first pass effects. Absorption is unaffected by food intake. Peak plasma concentrations of bisoprolol are attained within 2-4 hours and steady-state concentrations are achieved within 5 days of administration. In a pharmacokinetic study, the mean peak concentration of bisoprolol was 52 micrograms/L. Cmax at steady state concentrations of bisoprolol is 6421 ng/ml administered at 10 mg daily.
Route of Elimination
Bisoprolol is eliminated equally by both renal and hepatic pathways. About 50% of an oral dose is excreted unchanged in the urine with the remainder of the dose excreted as inactive bisoprolol metabolites. Under 2% of the ingested dose is found to be excreted in the feces.
Volume of Distribution
The volume of distribution of bisoprolol is 3.5 L/kg. The mean volume of distribution was found to be 230 L/kg in heart failure patients, which was similar to the volume of distribution in healthy patients. Bisoprolol is known to cross the placenta.
Clearance
Total body clearance in healthy patients was determined to be 14.2 L/h. In patients with renal impairment, clearance was reduced to 7.8 L/h. Hepatic dysfunction also reduced the clearance of bisoprolol.
Beagles were treated with bisoprolol, a beta 1-selective adrenoceptor antagonist, for 30 days with the following daily doses: oral: 30 mg/kg; conjunctival: 0.5% solution (approx. 0.04 mg/kg) and 5% solution (approx. 0.4 mg/kg). Drug concentrations were determined in plasma and various eye tissues on days 1, 16, and 30, and on day 59, i.e. on day 29 of the follow-up period. Bisoprolol concentrations in plasma and most eye tissues were considerably higher after oral than after conjunctival treatment. The highest tissue concentrations were observed in the iris (+ciliary body) and retina (+choroid) with tissue/plasma concentration ratios between 100 and 150 after oral and 1000 to 3000 after conjunctival instillation (5% solution). In plasma no accumulation of the drug was observed which is in accordance with its plasma half-life of 4 to 5 hr. In contrast to this, concentrations in the iris and retina increased from day 1 to day 16 and 30 by 3 to 8 times and the half-life of bisoprolol in these tissues was estimated to be between 3 to 5 days.
PMID:1983105 Buhring KU et al; Lens Eye Toxic Res 7 (3-4): 335-45 (1990)
The pharmacokinetic properties of bisoprolol-(14)C were studied in Wistar rats, beagle dogs, and Cynomolgus monkeys. Bisoprolol is well absorbed in these species; independent of the route of administration (IV or PO), 70-90% of the (14)C-dose was recovered in urine. Fecal excretion was approximately 20% in rats and less than 10% in dogs and monkeys. Rats excreted approximately 10% of the dose in bile after IV as well as after oral administration. The plasma half-life of the unchanged drug was approximately 1 hr in rats, 3 hr in monkeys, and 5 hr in dogs. The bioavailability was 40-50% in monkeys, approximately 80% in dogs, and 10% in rats. Studies in rats have shown that the drug is rapidly taken up by the tissues. After IV administration, high levels of radioactivity were found in lung, kidneys, liver, adrenals, spleen, pancreas, and salivary glands. After oral administration, the highest concentration occurred in the liver and kidneys. With the exception of plasma and liver, unchanged bisoprolol was the major radioactive constituent in all tissues studied. Both the blood-brain and placental barriers were penetrated, but only to a small degree. No accumulation of radioactivity in tissues was observed after repeated dosing (1 mg/kg/day). The metabolism of bisoprolol was studied in the same three animal species and in humans. The major metabolites are the products of O-dealkylation and subsequent oxidation to the corresponding carboxylic acids. The amount of bisoprolol excreted unchanged in the urine is 50-60% of the dose in humans, 30-40% in dogs, and approximately 10% in rats and monkeys.
PMID:2439794 Buhring KU et al; J Cardiovasc Pharmacol 8 (Suppl 11): S21-8 (1986)
The pharmacokinetics of bisoprolol (I) following an oral dose of 20 mg (14)C-labeled I solution, 10 mg tablet, and intravenous injection of 10 mg I were studied in 23 healthy volunteers (aged 37-53 yr). Mean elimination half-lives of 11 h for the unchanged I and 12 h for total radioactivity were observed. The enteral absorption of I was nearly complete. Fifty percent of the dose was eliminated renally as unchanged I and the other 50% metabolically, with subsequent renal excretion of the metabolites. Less than 2% of the dose was recovered from the feces. Intraindividual comparison of the pharmacokinetic data measured after oral or IV dose yielded an absolute bioavailability of 90%. Total and renal clearance were calculated as 15.6 l/h and 9.6 l/h, respectively. The volume of distribution was 226 l. Concomitant food intake did not influence the bioavailability of I.
Leopold G et al; J Clin Pharmacol 26 (Nov-Dec): 616-621 (1986)
We previously reported that renal function is partly responsible for the interindividual variability of the pharmacokinetics of bisoprolol. The aim of the present study was to examine the variability of bioavailability (F) of bisoprolol in routinely treated Japanese patients and intestinal absorption characteristics of the drug. We first analyzed the plasma concentration data of bisoprolol in 52 Japanese patients using a nonlinear mixed effects model. We also investigated the cellular uptake of bisoprolol using human intestinal epithelial LS180 cells. The oral clearance (CL/F) of bisoprolol in Japanese patients was positively correlated with the apparent volume of distribution (V/F), implying variable F. The uptake of bisoprolol in LS180 cells was temperature-dependent and saturable, and was significantly decreased in the presence of quinidine and diphenhydramine. In addition, the cellular uptake of bisoprolol dissolved in an acidic buffer was markedly less than that dissolved in a neutral buffer. These findings suggest that the rate/extent of the intestinal absorption of bisoprolol is another cause of the interindividual variability of the pharmacokinetics, and that the uptake of bisoprolol in intestinal epithelial cells is highly pH-dependent and also variable.
PMID:23719964 Ishida K et al; Drug Metab Pharmacokinet 28 (6): 491-6 (2013)
For more Absorption, Distribution and Excretion (Complete) data for Bisoprolol (9 total), please visit the HSDB record page.
About 50% of a single bisoprolol dose is metabolized mainly by the enzyme CYP3A4 to inactive metabolites.
... In humans, the known metabolites are labile or have no known pharmacologic activity. ... Bisoprolol fumarate is not metabolized by cytochrome P450 II D6 (debrisoquin hydroxylase).
NIH; DailyMed. Current Medication Information for Zebeta (Bisoprolol Fumarate) Tablet, Film Coated (Updated: January 2016). Available from, as of January 24, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a11548a0-9c0f-4729-907c-75d8f99a6c85
The plasma concentrations and urinary excretions of bisoprolol enantiomers in four Japanese male healthy volunteers after a single oral administration of 20 mg of racemic bisoprolol were evaluated. The AUC(infinity) and elimination half-life of (S)-(-)-bisoprolol were slightly larger than those of (R)-(+)-bisoprolol in all subjects. The metabolic clearance of (R)-(+)-bisoprolol was significantly (P < 0.05) larger than that of (S)-(-)-bisoprolol (S/R ratio: 0.79+/-0.03), although the difference was small. In contrast, no stereoselective in vitro protein binding of bisoprolol in human plasma was found. An in vitro metabolic study using recombinant human cytochrome P450 (CYP) isoforms indicated that oxidation of both bisoprolol enantiomers was catalyzed by the two isoforms, CYP2D6 and CYP3A4. CYP2D6 metabolized bisoprolol stereoselectively (R > S), whereas the metabolism of bisoprolol by CYP3A4 was not stereoselective. The S/R ratio of the mean clearance due to renal tubular secretion was 0.68, indicating a moderate degree of stereoselective renal tubular secretion. These findings taken together suggest that the small differences in the pharmacokinetics between (S)-(-)- and (R)-(+)-bisoprolol are mainly due to the stereoselectivity in the intrinsic metabolic clearance by CYP2D6 and renal tubular secretion.
PMID:9523980 Horikiri Y et al; J Pharm Sci 87 (3): 289-94 (1998)
The pharmacokinetic properties of bisoprolol-(14)C were studied in Wistar rats, beagle dogs, and Cynomolgus monkeys. ... The metabolism of bisoprolol was studied in the same three animal species and in humans. The major metabolites are the products of O-dealkylation and subsequent oxidation to the corresponding carboxylic acids. ...
PMID:2439794 Buhring KU et al; J Cardiovasc Pharmacol 8 (Suppl 11): S21-8 (1986)
A pharmacokinetic study in 12 healthy individuals determined the mean plasma half-life of bisoprolol to be 10-12 hours. Another study comprised of healthy patients determined the elimination half-life to be approximately 10 hours. Renal impairment increased the half-life to 18.5 hours.
In patients with cirrhosis of the liver, the elimination of Zebeta (bisoprolol fumarate) is more variable in rate and significantly slower than that in healthy subjects, with plasma half-life ranging from 8.3 to 21.7 hours.
NIH; DailyMed. Current Medication Information for Zebeta (Bisoprolol Fumarate) Tablet, Film Coated (Updated: January 2016). Available from, as of January 24, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a11548a0-9c0f-4729-907c-75d8f99a6c85
In subjects with creatinine clearance less than 40 mL/min, the plasma half-life is increased approximately threefold compared to healthy subjects.
NIH; DailyMed. Current Medication Information for Zebeta (Bisoprolol Fumarate) Tablet, Film Coated (Updated: January 2016). Available from, as of January 24, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a11548a0-9c0f-4729-907c-75d8f99a6c85
The plasma elimination half-life is 9-12 hours and is slightly longer in elderly patients, in part because of decreased renal function in that population.
NIH; DailyMed. Current Medication Information for Zebeta (Bisoprolol Fumarate) Tablet, Film Coated (Updated: January 2016). Available from, as of January 24, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a11548a0-9c0f-4729-907c-75d8f99a6c85
The pharmacokinetic properties of bisoprolol-(14)C were studied in Wistar rats, beagle dogs, and Cynomolgus monkeys. ... The plasma half-life of the unchanged drug was approximately 1 hr in rats, 3 hr in monkeys, and 5 hr in dogs.
PMID:2439794 Buhring KU et al; J Cardiovasc Pharmacol 8 (Suppl 11): S21-8 (1986)
In dogs, bisoprolol has ... a half life of 4 hours
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 74
Though the mechanism of action of bisoprolol has not been fully elucidated in hypertension, it is thought that therapeutic effects are achieved through the antagonism of -1adrenoceptors to result in lower cardiac output. Bisoprolol is a competitive, cardioselective 1-adrenergic antagonist. When 1-receptors (located mainly in the heart) are activated by adrenergic neurotransmitters such as epinephrine, both the blood pressure and heart rate increase, leading to greater cardiovascular work, increasing the demand for oxygen. Bisoprolol reduces cardiac workload by decreasing contractility and the need for oxygen through competitive inhibition of 1-adrenergic receptors. Bisoprolol is also thought to reduce the output of renin in the kidneys, which normally increases blood pressure. Additionally, some central nervous system effects of bisoprolol may include diminishing sympathetic nervous system output from the brain, decreasing blood pressure and heart rate.