


1. 673-06-3
2. H-d-phe-oh
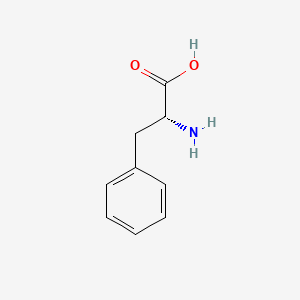
3. (2r)-2-amino-3-phenylpropanoic Acid
4. Phenylalanine D-form
5. Sabiden
6. D-phe
7. Alanine, Phenyl-, D-
8. (r)-2-amino-3-phenylpropanoic Acid
9. Nci-c60195
10. Phenylalanine,d-
11. D-alpha-amino-beta-phenylpropionic Acid
12. D-(+)-phenylalanine
13. (r)-2-amino-3-phenylpropionic Acid
14. 032k16vrcu
15. Chebi:16998
16. Mfcd00004270
17. D-.beta.-phenylalanine
18. Ccris 6267
19. Einecs 211-603-5
20. Nsc 25005
21. Unii-032k16vrcu
22. Nsc-25005
23. Dphe
24. L-3-phenylalanine
25. Hdpheoh
26. (d)-phenylalanine
27. Phenylalanine,(s)
28. Spectrum_001725
29. Spectrum2_001558
30. Spectrum4_000865
31. Spectrum5_001137
32. (r)-phenylalanine
33. Phenylalanine, D-
34. D-phenylalanine, >=99%
35. Schembl92744
36. Kbiogr_001529
37. Kbioss_002205
38. Divk1c_000453
39. Spectrum1503391
40. Spbio_001436
41. Chembl379630
42. Gtpl5797
43. Zinc1927
44. Dtxsid4025876
45. Bdbm36161
46. Hms501g15
47. Kbio1_000453
48. Kbio2_002205
49. Kbio2_004773
50. Kbio2_007341
51. D-phenylalanine [who-dd]
52. D-.beta.-phenyl-.alpha.-alanine
53. Ninds_000453
54. Phenylalanine D-form [mi]
55. Hms1922c08
56. Pharmakon1600-01503391
57. Act08578
58. Hy-y0079
59. D-phenylalanine, >=98% (hplc)
60. Ccg-39336
61. Nsc758460
62. S4153
63. Akos007930513
64. Ac-8664
65. Am83526
66. Cs-w020009
67. Db02556
68. Nsc-758460
69. Idi1_000453
70. Ncgc00163338-01
71. Ncgc00163338-03
72. Ac-11292
73. As-11997
74. Sbi-0051820.p002
75. P0135
76. En300-60166
77. 73p063
78. A20666
79. C02265
80. M02934
81. Ab00052351_02
82. Ab00052351_03
83. Sr-01000872765
84. D-phenylalanine, Vetec(tm) Reagent Grade, >=98%
85. J-300203
86. Q-101646
87. Sr-01000872765-1
88. Q26841253
89. Z1696844707
90. 4c53b247-2fe4-4464-92c0-9f3782a08966
91. D-alpha-amino-beta-phenylpropionic Acid;d-beta-phenyl-alpha-aminopropionic Acid
| Molecular Weight | 165.19 g/mol |
|---|---|
| Molecular Formula | C9H11NO2 |
| XLogP3 | -1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 165.078978594 g/mol |
| Monoisotopic Mass | 165.078978594 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 153 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |