
1. A 43818
2. A-43818
3. A43818
4. Acetate, Leuprolide
5. Enantone
6. Leuprolide
7. Leuprolide Monoacetate
8. Leuprolide, (dl-leu)-isomer
9. Leuprolide, (l-leu)-isomer
10. Leuprorelin
11. Lupron
12. Monoacetate, Leuprolide
13. Tap 144
14. Tap-144
15. Tap144
1. Leuprorelin Acetate
2. Lupron
3. 74381-53-6
4. Carcinil
5. Enantone
6. Lucrin
7. Eligard
8. Leuplin
9. Viadur
10. Lupron Depot
11. Tap-144
12. Abbott-43818
13. Prostap
14. Leuprolide Monoacetate
15. Trenantone
16. Fensolvi
17. Lupaneta
18. Tap-144-sr
19. Lupron Depot-ped
20. Lutrate Depot
21. Leuporelin Acetate
22. A-43818
23. Leuprolide (acetate)
24. Chebi:63597
25. 53714-56-0
26. 37jns02e7v
27. Leuprolide Acetate [usan]
28. Leuprin
29. Procrin
30. Leuplin Depot
31. Procren Depot
32. Uno-enantone
33. Depo-lupron
34. Lupron Ped
35. Leuprorelin Acetate [jan]
36. Leuprolide Acetate Depot
37. Abbott 43818
38. Lupron Depot-3
39. Lupron Depot-4
40. Unii-37jns02e7v
41. Enanton
42. Lutrate
43. Onectyl
44. Mfcd00072080
45. Leuprolide Acetate [usan:usp]
46. Lupron Depot Ped
47. Leuprolide Acetate Salt
48. Des-gly10-[d-leu6]-lh-rh Ethylamide
49. Ncgc00183364-01
50. Schembl3174
51. Dsstox_cid_28935
52. Dsstox_rid_83201
53. Dsstox_gsid_49009
54. Mls000028695
55. Chembl1200775
56. Dtxsid7049009
57. Leuprolide Acetate [vandf]
58. Leuprolide Monoacetate [mi]
59. Leuprolide Acetate [usp-rs]
60. Leuprorelin Acetate [mart.]
61. Tox21_113507
62. Bdbm50247891
63. Leuprorelin Acetate [who-dd]
64. S3718
65. Akos015895632
66. Akos030485977
67. Ccg-270666
68. Cs-1434
69. Leuprolide Acetate [orange Book]
70. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tryosyl-d-leucyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide Monoacetate (salt)
71. Ac-28731
72. Hy-13665
73. Leuprolide Acetate [usp Monograph]
74. Smr000058945
75. Cas-74381-53-6
76. L0249
77. (des-gly10,d-leu6,pro-nhet9)-lhrh Acetate
78. Leuprolide Acetate (53714-56-0 Free Base)
79. 381l536
80. 743l536
81. A829746
82. A838107
83. Pyr-his-trp-ser-tyr-d-leu-leu-arg-pro-nhet.hoac
84. Q27104908
85. Pglu-his-trp-ser-tyr-d-leu-leu-arg-pro-nhc2h5 Acetate Salt
86. 1-9-leutenizing Hormone-releasing Factor (swine), 6-d-leucine-9-(n-ethyl-l-prolinamide)-, Monoacetate (salt)
87. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tryosyl-d-leucyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide Acetate (salt)
88. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tryosyl-d-leucyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide Monoacetate
89. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-leucyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide--acetic Acid (1/1)
90. 88793-81-1
91. Acetic Acid; N-[2-[[2-[[2-[[2-[[1-[[1-[[1-[2-(ethylcarbamoyl)pyrrolidine-1-carbonyl]-4-guanidino-butyl]carbamoyl]-2-methyl-propyl]carbamoyl]-2-methyl-propyl]amino]-1-[(4-hydroxyphenyl)methyl]-2-oxo-ethyl]amino]-1-(hydroxymethyl)-2-oxo-ethyl]amino]-1-(1h-i
92. Acetic Acid; N-[2-[[2-[[2-[[2-[[1-[[1-[[1-[2-(ethylcarbamoyl)pyrrolidine-1-carbonyl]-4-guanidino-butyl]carbamoyl]-3-methyl-butyl]carbamoyl]-3-methyl-butyl]amino]-1-[(4-hydroxyphenyl)methyl]-2-oxo-ethyl]amino]-1-(hydroxymethyl)-2-oxo-ethyl]amino]-1-(1h-ind;leuprolide Acetate
93. L-prolinamide, 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-leucyl-l-leucyl-l-arginyl-n-ethyl-, Acetate (1:1)
94. Luteinizing Hormone-releasing Factor (pig), 6-d-leucine-9-(n-ethyl-l-prolinamide)-10-deglycinamide-, Monoacetate (salt)
95. Luteinizing Hormone-releasing Factor, 6-d-leucine-9-(n-ethyl-l-prolinamide)-10-deglycinamide Acetate (salt)
| Molecular Weight | 1269.4 g/mol |
|---|---|
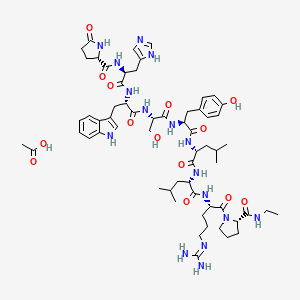
| Molecular Formula | C61H88N16O14 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 32 |
| Exact Mass | 1268.66659154 g/mol |
| Monoisotopic Mass | 1268.66659154 g/mol |
| Topological Polar Surface Area | 469 Ų |
| Heavy Atom Count | 91 |
| Formal Charge | 0 |
| Complexity | 2420 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 5 | |
|---|---|
| Drug Name | ELIGARD |
| Active Ingredient | LEUPROLIDE ACETATE |
| Company | TOLMAR THERAP (Application Number: N021343. Patents: 6565874, 6626870, 6773714, 9254307); TOLMAR THERAP (Application Number: N021379. Patents: 6565874, 6626870, 6773714, 8470359, 8486455, 8840916, 9283282, 9539333); TOLMAR THERAP (Application Number: N021488. Patents: 6565874, 6626870, 6773714, 8470359, 8486455, 8840916, 9283282, 9539333); TOLMAR THERAP (Application Number: N021731. Patents: 6565874, 6626870, 6773714, 8470359, 8486455, 8840916, 9283282, 9539333) |
| 2 of 5 | |
|---|---|
| Drug Name | LEUPROLIDE ACETATE |
| Active Ingredient | LEUPROLIDE ACETATE |
| Company | SANDOZ (Application Number: A074728); SUN PHARMA GLOBAL (Application Number: A078885); TEVA PHARMS USA (Application Number: A075471) |
| 3 of 5 | |
|---|---|
| Drug Name | LUPRON DEPOT-PED |
| Active Ingredient | LEUPROLIDE ACETATE |
| Company | ABBVIE ENDOCRINE INC (Application Number: N020263) |
| 4 of 5 | |
|---|---|
| Drug Name | LUPRON DEPOT |
| Active Ingredient | LEUPROLIDE ACETATE |
| Company | ABBVIE ENDOCRINE INC (Application Number: N019732); ABBVIE ENDOCRINE INC (Application Number: N020011); ABBVIE ENDOCRINE INC (Application Number: N020517. Patents: 7429559, 8815801, 8921326); ABBVIE ENDOCRINE INC (Application Number: N020708) |
| 5 of 5 | |
|---|---|
| Drug Name | LUPANETA PACK |
| Active Ingredient | LEUPROLIDE ACETATE; NORETHINDRONE ACETATE |
| Company | ABBVIE ENDOCRINE (Application Number: N203696) |
Antineoplastic Agents, Hormonal; Fertility Agents, Female
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Leuprolide is indicated for the palliative treatment of advanced prostatic cancer, especially as an alternative to orchiectomy or estrogen administration. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
Leuprolide is indicated for management of endometriosis, including pain relief and reduction of endometriotic lesions. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
Leuprolide is about 30 times more active than natural gonadotropin-releasing hormone ... and 100 times more active than gonadorelin.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1354
For more Therapeutic Uses (Complete) data for LEUPROLIDE (15 total), please visit the HSDB record page.
Patients sensitive to other synthetic gonadotropin-releasing hormone analogs may also be sensitive to leuprolide.
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
In males: Suppression of testosterone secretion results in impairment of fertility. Although it is not known whether fertility is restored after leuprolide is withdrawn, reversal of fertility suppression does occur after withdrawal of similar analogs.
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
Leuprolide is not recommended during pregnancy. Because the effects on fetal mortality would logically result from the hormonal effects of leuprolide, it can be concluded that there is a risk of spontaneous abortion if leuprolide is administered during pregnancy.
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
It is not known whether leuprolide passes into breast milk. However, because of potential adverse effects in the infant, breast-feeding is usually not recommended during treatment with leuprolide.
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
For more Drug Warnings (Complete) data for LEUPROLIDE (14 total), please visit the HSDB record page.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Fertility Agents, Female
Compounds which increase the capacity to conceive in females. (See all compounds classified as Fertility Agents, Female.)
Bioavailablity after intramuscular injection of the depot formulation is estimated to be about 90%.
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
The pharmacological effects of leuprolide acetate depot microspheres were studied in rats and dogs following subcutaneous and intramuscular injection. After injection the microspheres provided similar linear drug release and sustained serum drug levels for 3 months. Persistent suppression of serum luteinizing hormone, follicle stimulating hormone in rats, and testosterone in rats and dogs for over 16 wk was achieved with microspheres at a dose of 100 ug/kg/day in rats and 25.6 ug/kg/day in dogs. Responses upon periodic challenge tests revealed that a single injection of microspheres dramatically suppressed the function of the pituitary-gonadal system for 15 wks in rats. The growth of genital organs was also suppressed dose-dependently for over 3 months. It was concluded that persistent pharmacological effects are obtained with an injection of leuprolide 3-month depot microspheres.
PMID:7971724 Okada H et al; Pharm Res 11 (Aug): 1199-1203 (1994)
The effect of formulation adjuvants on the absorption of leuprolide acetate after intraduodenal injection and oral administration to male castrate rats is reported. Absorption was low, approximately 0.01% and 0.08% by oral and intraduodenal administration, respectively, compared with intravenous controls. An aqueous formulation and a water-in-oil emulsion of a lipophilic salt, a decane sulfonic acid derivative of leuprolide gave intraduodenal bioavailabilities of approximately 0.2% and 1% respectively. Evaluation of formulation effects on the oral absorption of the drug showed that lipophilicity, surfactant, and vehicle properties significantly affected intraduodenal absorption of leuprolide. Absolute bioavailability of the drug in typical emulsion systems ranged from approximately 3-10% and represented an improvement of about 100-fold in gastrointestinal bioavailability of this peptide. The implications of these findings relative to the effect of formulation adjuvants on oral absorption of leuprolide and other peptides following intraduodenal administration are discussed.
Adjel A et al; L Drug Target 1 (3): 251-8 (1993)
The bioavailability of leuprolide acetate was studied in rats and in healthy males (ages 19-39 yr) after inhalation and intranasal administration, compared with intravenous and subcutaneous injection. Intranasal bioavailability in rats was significantly increased by alpha-cyclodextrin, eidetic acid, and solution volume. Intra-animal variability was 30-60% and absorption ranged from 8 to 46% compared with intravenous controls. In humans, the subcutaneous injection was 94% bioavailable compared with intravenous. Intranasal bioavailability averaged 2.4%, with significant intersubject variability. Plasma peak concentrations for one and 3 mg dosages were 0.24-1.6 and 0.1-11 ng/ml, respectively. Mean plasma peak concentrations of one mg aerosol and 2 mg suspension aerosols, respectively. Bioavailability of suspension aerosols was fourfold greater than that of the solution aerosol. /Leuprolide acetate/
PMID:1553349 Adjel A et al; Pharm Res 9 (Feb): 244-9 (1992)
Like naturally occurring luteinizing hormone-releasing hormone, initial or intermittent administration of leuprolide stimulates release of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary.
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
Luteinizing hormone and follicle-stimulating hormone release from the anterior pituitary transiently increases testosterone concentration in males. However, continuous administration of leuprolide in the treatment of prostatic carcinoma suppresses secretion of gonadotropin-releasing hormone, with a resultant fall in testosterone concentrations and a "medical castration".
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708
Initial stimulation of gonadotropins form the anterior pituitary is followed by prolonged suppression. Gonadotropin release from the anterior pituitary transiently increases estrone and estradiol concentrations in females. However, continuous administration of leuprolide in the treatment of endometriosis produces a fall in estrogens to postmenopausal levels. As a consequence of suppression of ovarian function, both normal and ectopic endometrial tissues become inactive and atrophic. As a result, amenorrhea occurs.
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 1708