
1. 83-67-0
2. 3,7-dimethylxanthine
3. Diurobromine
4. Theosalvose
5. Santheose
6. Teobromin
7. Theostene
8. Thesodate
9. 3,7-dimethylpurine-2,6-dione
10. Thesal
11. 3,7-dimethyl-3,7-dihydro-1h-purine-2,6-dione
12. Theobromin
13. 3,7-dihydro-3,7-dimethyl-1h-purine-2,6-dione
14. Xantheose
15. Xanthine, 3,7-dimethyl-
16. 2,6-dihydroxy-3,7-dimethylpurine
17. Fema No. 3591
18. 1h-purine-2,6-dione, 3,7-dihydro-3,7-dimethyl-
19. Sc 15090
20. Theobrominum
21. Nsc 5039
22. 2,6-dihydroxy-3,7-dimethyl-purine
23. Mfcd00022830
24. Brn 0016464
25. 519-41-5
26. 3,7-dimethyl-2,3,6,7-tetrahydro-1h-purine-2,6-dione
27. Nsc-5039
28. Chembl1114
29. Obd445wz5p
30. Chebi:28946
31. Nsc5039
32. 3,7-dimethyl-1h-purine-2,6-dione
33. Cas-83-67-0
34. Ncgc00016023-11
35. Sc-15090
36. Dsstox_cid_6132
37. 3,7-dimethyl-1h-purine-2,6(3h,7h)-dione
38. Dsstox_rid_78030
39. Dsstox_gsid_26132
40. Theobromine (natural)
41. 7-dimethylxanthine
42. Ccris 2350
43. Hsdb 7332
44. Sr-01000000069
45. Einecs 201-494-2
46. Unii-obd445wz5p
47. Theobromine [inn:ban:nf]
48. Theobromine;
49. Cocoa Theobromine
50. 37t
51. Theobromine (3,7-dimethylxanthine)
52. Prestwick_1054
53. Theobromine(20%)
54. 3,7-dimethylxanthin
55. Spectrum_000053
56. 3, 7-dimethylxanthine
57. Theobromine [mi]
58. Prestwick0_000874
59. Prestwick1_000874
60. Prestwick2_000874
61. Prestwick3_000874
62. Spectrum2_000985
63. Spectrum3_000279
64. Spectrum4_000403
65. Spectrum5_001387
66. Lopac-t-4500
67. Theobromine [fhfi]
68. Theobromine [hsdb]
69. Theobromine [iarc]
70. Theobromine [inci]
71. Theobromine [vandf]
72. Theobrominum [hpus]
73. Schembl3184
74. Theobromine, >=98.0%
75. 3,7-dimethyl-1,3,7-trihydropurine-2,6-dione
76. Theobromine [mart.]
77. Lopac0_001187
78. Bspbio_000947
79. Bspbio_001758
80. Kbiogr_000666
81. Kbioss_000433
82. Theobromine [who-dd]
83. 5-26-13-00553 (beilstein Handbook Reference)
84. Mls000028407
85. Divk1c_000611
86. Spectrum1500649
87. Spbio_001049
88. Spbio_002868
89. Bpbio1_001043
90. Zinc2151
91. Theobromine, Analytical Standard
92. Dtxsid9026132
93. Hms501o13
94. Kbio1_000611
95. Kbio2_000433
96. Kbio2_003001
97. Kbio2_005569
98. Kbio3_001258
99. Yapqbxqyljrxsa-uhfffaoysa-
100. Theobromine [ep Impurity]
101. Ninds_000611
102. Hms1570p09
103. Hms1921o13
104. Hms2092g04
105. Hms2097p09
106. Hms3263n15
107. Hms3714p09
108. Pharmakon1600-01500649
109. Hy-n0138
110. Theobromine 0.1 Mg/ml In Methanol
111. Theobromine, >=98.0% (hplc)
112. Tox21_110284
113. Tox21_300016
114. Tox21_501187
115. Bbl034679
116. Bdbm50014260
117. Ccg-40078
118. Nsc757407
119. Pdsp1_001017
120. Pdsp2_001001
121. S2368
122. Stl419465
123. Akos000121558
124. Tox21_110284_1
125. 5-26-13-00553 (beilstein)
126. Cs-7972
127. Db01412
128. Lp01187
129. Nsc-757407
130. Sdccgmls-0002875.p003
131. Sdccgsbi-0051154.p004
132. Idi1_000611
133. Ncgc00016023-01
134. Ncgc00016023-02
135. Ncgc00016023-03
136. Ncgc00016023-04
137. Ncgc00016023-05
138. Ncgc00016023-06
139. Ncgc00016023-07
140. Ncgc00016023-08
141. Ncgc00016023-09
142. Ncgc00016023-10
143. Ncgc00016023-12
144. Ncgc00016023-13
145. Ncgc00016023-14
146. Ncgc00016023-15
147. Ncgc00016023-17
148. Ncgc00016023-18
149. Ncgc00016023-26
150. Ncgc00024123-03
151. Ncgc00024123-04
152. Ncgc00024123-05
153. Ncgc00024123-06
154. Ncgc00024123-07
155. Ncgc00024123-08
156. Ncgc00179030-01
157. Ncgc00179030-02
158. Ncgc00253943-01
159. Ncgc00261872-01
160. Wln: T56 Bn Dn Fnvmvj B1 F1
161. Ac-11381
162. Ac-34381
163. As-13904
164. Smr000058357
165. Sy048379
166. Sbi-0051154.p003
167. 1h-purine-2, 3,7-dihydro-3,7-dimethyl-
168. Ab00052141
169. Eu-0101187
170. Ft-0660435
171. Ft-0675138
172. N1573
173. T0178
174. C07480
175. D71206
176. Pentoxifylline Impurity A [ep Impurity]
177. T 4500
178. Ab00052141_12
179. Q206844
180. 3,7-dimethyl-3,7-dihydro-1h-purine-2,6-dione #
181. Q-100848
182. Sr-01000000069-2
183. Sr-01000000069-4
184. Sr-01000000069-7
185. Sr-01000000069-8
186. Sr-01000000069-9
187. Brd-k34888156-001-08-8
188. Caffeine Monohydrate Impurity D [ep Impurity]
189. Cec63cca-3b4b-4f4f-92c1-1789df3c880a
190. Sr-01000000069-10
191. Z56347209
192. 1h-purine-2,6-dione,3,7-dihydro-3,7- Dimethyl- (9ci)
193. Theobromine, European Pharmacopoeia (ep) Reference Standard
194. 3,7-dimethylxanthine; 3,7-dimethylpurine-2,6-dione
195. Theobromine, Pharmaceutical Secondary Standard; Certified Reference Material
196. Theobromine Solution, 100 Mug/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 180.16 g/mol |
|---|---|
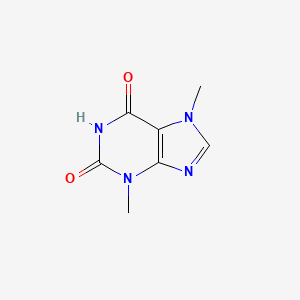
| Molecular Formula | C7H8N4O2 |
| XLogP3 | -0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 180.06472551 g/mol |
| Monoisotopic Mass | 180.06472551 g/mol |
| Topological Polar Surface Area | 67.2 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 267 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Diuretic, bronchodilator, cardiotonic. /Former use/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1654
Formerly, theobromine and its derivatives were used in diuretics, myocardial stimulants, vasodilators and smooth muscle relaxants. Theobromine salts (calcium salicylate, sodium salicylate and sodium acetate) were used previously to dilate coronary arteries at doses of 300 to 600 mg per day. There is no current therapeutic use of theobromine. /Former use/
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 422 (1991)
VET: Diuretic, myocardial stimulant, vasodilator. /Former use/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1654
/EXPL THER/ Cough is a common and protective reflex, but persistent coughing is debilitating and impairs quality of life. Antitussive treatment using opioids is limited by unacceptable side effects, and there is a great need for more effective remedies. The present study demonstrates that theobromine, a methylxanthine derivative present in cocoa, effectively inhibits citric acid-induced cough in guinea-pigs in vivo. Furthermore, in a randomized, double-blind, placebo-controlled study in man, theobromine suppresses capsaicin-induced cough with no adverse effects. We also demonstrate that theobromine directly inhibits capsaicin-induced sensory nerve depolarization of guinea-pig and human vagus nerve suggestive of an inhibitory effect on afferent nerve activation. These data indicate the actions of theobromine appear to be peripherally mediated. We conclude theobromine is a novel and promising treatment, which may form the basis for a new class of antitussive drugs.
PMID:15548587 Usmani OS et al; FASEB J 19 (2): 231-3 (2005)
theobromine is used as a vasodilator, a diuretic, and heart stimulant. And similar to caffeine, it may be useful in management of fatigue and orthostatic hypotension.
Theobromine, a xanthine derivative like caffeine and the bronchodilator theophylline, is used as a CNS stimulant, mild diuretic, and respiratory stimulant (in neonates with apnea of prematurity).
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
C - Cardiovascular system
C03 - Diuretics
C03B - Low-ceiling diuretics, excl. thiazides
C03BD - Xanthine derivatives
C03BD01 - Theobromine
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03D - Other systemic drugs for obstructive airway diseases
R03DA - Xanthines
R03DA07 - Theobromine
The ratio of brain:blood theobromine concentrations decreased continuously from 0.96 at birth to 0.60 in 30-day-old rats. After 24 hr, no organ accumulation of theobromine or its metabolites could be seen in adult animals.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 425 (1991)
Theobromine is absorbed and distributed rapidly after oral administration to rats and equilibrates freely between plasma and testicular fluid.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 425 (1991)
Similar kinetic parameters were observed in male and female rabbits when theobromine was administered intravenously or orally at doses of 1 and 5 mg/kg bw, with complete gastrointestinal absorption. A reduction in the absorption rate constant was seen in rabbits when the dose was increased from 10 to 100 mg/kg bw. In spite of delayed gastrointestinal absorption at high doses, probably due to the low solubility of the compound, the absolute bioavailability of theobromine approached 100%. Labelled theobromine was almost completely absorbed after oral administration (1-6 mg/kg); the peak blood level tended to appear later with larger doses.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 425 (1991)
When theobromine was given as a single oral dose of 15-50 mg/kg bw to male dogs, peak plasma concentrations, with considerable individual variations, were observed within 3 hr. With a higher dose (150 mg/kg bw), the peak plasma concentrations were attained 14-16 hr later, showing delayed intestinal absorption. In rats, plasma protein binding was very low (8-17%) after oral administration of 1-100 mg/kg bw theobromine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 425 (1991)
For more Absorption, Distribution and Excretion (Complete) data for 3,7-Dimethylxanthine (17 total), please visit the HSDB record page.
Pregnancy and increased doses of theobromine were shown to modify theobromine metabolism. At a dose of 50 mg/kg bw, pregnant rabbits excreted more unchanged theobromine in the urine (51% versus 35%). Pregnant rats excreted a higher percentage of a 5 mg/kg dose as unchanged theobromine (53%) than non-pregnant rats (39%); this difference disappeared at the saturation dose (100 mg/kg), when unchanged theobromine corresponded to about 60% of the dose in the urine of both pregnant and non-pregnant animals. Rats given 100 mg/kg excreted more unchanged theobromine than those given 1 mg/kg (73% versus 51%), and showed a corresponding relative decrease in excretion of its uracil metabolite, 6-amino-5-(N-methylformylamino)-1-methyluracil (16% versus 28%).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 428 (1991)
The compounds identified in bile of phenobarbital-treated rats were 3,7-dimethyluric acid (64-76% of biliary radioactivity), dimethylallantoin (5-8%), 6-amino-5-(N-methylformylamino)- 1-methyluracil (10-17%) and theobromine (8-10%). In 3-methylcholanthrene-treated rats, urinary elimination of unchanged theobromine was reduced from 23-27% to only 2%, while excretion of 6-amino-5-(N-methylformylamino)-1- methyluracil was significantly increased. Only 3,7-dimethyluric acid was produced by liver microsomal incubation in control rats while phenobarbital and 3-methylcholanthrene pretreatment enhanced the biotransformation resulting in the production of all metabolites found in vivo as well as unknown polar compounds.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 426 (1991)
6-Amino-5-(N-methylformylamino)-1-methyluracil is quantitatively the most important theobromine metabolite in rats, accounting for 20-35% of urinary metabolites. The majority of theobromine-derived radioactivity in the feces of rats could be accounted for by 3,7-dimethyluric acid. The most extensive metabolism of theobromine was observed in rabbits and mice; male mice converted theobromine more extensively into this metabolite than did female mice. In contrast, oxidation of theobromine to 3,7-dimethyluric acid was significantly greater in female than in male rats. Rabbits and dogs metabolized theobromine primarily to 7-methylxanthine and 3-methylxanthine, respectively, and dogs excreted small quantities of an unidentified metabolite.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 426 (1991)
As a metabolite of caffeine, theobromine has been detected in variable amounts in plasma and urine of humans and different animal species.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 425 (1991)
For more Metabolism/Metabolites (Complete) data for 3,7-Dimethylxanthine (11 total), please visit the HSDB record page.
Theobromine has known human metabolites that include 3,7-Dimethyluric acid, 3-Methylxanthine, and 7-Methylxanthine.
Theobromine is a known human metabolite of caffeine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean half-time of theobromine in human serum ranged from 6.1 to 10 hr.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 432 (1991)
The disposition half-life of theobromine averaged 7.1 +/- 2.1 hours ...
PMID:894424 Resman BH et al; J Pediatr 91 (3): 477-80 (1977)
In dogs, an average plasma half-time of 17.5 hr was reported after single oral doses of theobromine ranging from 15 to 150 mg/kg bw. In rabbits, the mean elimination half-time was 4.3-5.6 hr for doses ranging from 1 to 100 mg/kg bw.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 426 (1991)
Theobromine stimulates medullary, vagal, vasomotor, and respiratory centers, promoting bradycardia, vasoconstriction, and increased respiratory rate. This action was previously believed to be due primarily to increased intracellular cyclic 3′,5′-adenosine monophosphate (cyclic AMP) following inhibition of phosphodiesterase, the enzyme that degrades cyclic AMP. It is now thought that xanthines such as caffeine and theobromine act as antagonist at adenosine-receptors within the plasma membrane of virtually every cell. As adenosine acts as an autocoid, inhibiting the release of neurotransmitters from presynaptic sites but augmenting the actions of norepinephrine or angiotensin, antagonism of adenosine receptors promotes neurotransmitter release. This explains the stimulatory effects of xanthine derivatives such as theobromine and caffeine. Blockade of the adenosine A1 receptor in the heart leads to the accelerated, pronounced "pounding" of the heart upon caffeine intake.