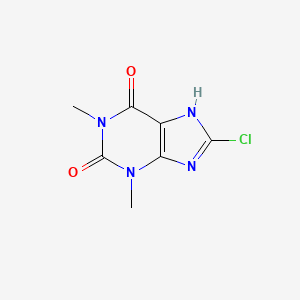

1. 8-chlorotheophylline, Sodium Salt
2. S8ct
3. Sodium-8-chlorotheophylline
1. 85-18-7
2. 1,3-dimethyl-8-chloroxanthine
3. 8-chloro-1,3-dimethyl-1h-purine-2,6(3h,7h)-dione
4. 8-chloro-1,3-dimethyl-7h-purine-2,6-dione
5. Chlortheophylline
6. Theophylline, 8-chloro-
7. 8-chloro-theophylline
8. 1h-purine-2,6-dione, 8-chloro-3,7-dihydro-1,3-dimethyl-
9. 8-chloro-1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione
10. Chlorotheophylline
11. Nsc 6113
12. 8-chloro-3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione
13. Ge2ua340fm
14. Chembl88611
15. Chebi:59771
16. 8-chloro-1,3-dimethyl-2,6(1h,3h)-purinedione
17. Nsc-6113
18. 8-chloro-1,3-dimethyl-3,7-dihydro-purine-2,6-dione
19. 8-chlortheophyllin
20. 8-chloro-1,3-dimethyl-2,6-purinedione
21. Einecs 201-590-4
22. Unii-ge2ua340fm
23. 8-chloro-1,3-dimethyl-3,9-dihydro-1h-purine-2,6-dione
24. 8-chorotheophylline
25. 8-chlorotheophyline
26. Mfcd00005581
27. 2uy3
28. 8-chloro-1,3-dimethyl-2,3,6,7-tetrahydro-1h-purine-2,6-dione
29. 8-chlortheophylline
30. Ec 201-590-4
31. Oprea1_038673
32. Oprea1_741931
33. Schembl411139
34. Amy493
35. Dtxsid5043764
36. 1,3-dimethyl 8-chloro Xanthine
37. Nsc6113
38. Bbl009700
39. Bdbm50331852
40. Stk701164
41. 8-chlortheophylline [who-dd]
42. 8-chlorotheophylline [usp-rs]
43. Akos000119705
44. Akos005521865
45. Zinc100018165
46. Db14132
47. As-67718
48. Db-056856
49. Ft-0631394
50. Pamabrom Related Compound A [usp-rs]
51. D97697
52. Ab01563382_01
53. A841253
54. Sr-01000479303
55. Q4644272
56. Sr-01000479303-1
57. W-104090
58. 1h-purine-2, 8-chloro-3,7-dihydro-1,3-dimethyl-
59. 8-chloro-1,3-dimethyl-3,7-dihydropurine-2,6-dione
60. 8-chloro-1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione #
61. 8-chloro-1,3-dimethyl-3,9-dihydro-1h-purine-2,6-dione, Aldrichcpr
62. H33
| Molecular Weight | 214.61 g/mol |
|---|---|
| Molecular Formula | C7H7ClN4O2 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 214.0257532 g/mol |
| Monoisotopic Mass | 214.0257532 g/mol |
| Topological Polar Surface Area | 69.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 297 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
When used in combination with [DB01075] as the antiemetic [DB00985], 8-chlorotheophylline is indicated for the prevention and treatment of nausea, vomiting, or vertigo of motion sickness.
8-chlorotheophylline produces a number of effects including nervousness, restlessness, insomnia, headache, and nausea, which are primarily attributed to its ability to block the adenosine receptor. Because adenosine causes a decrease in neuronal firing, blockade of the adenosine receptor causes the reverse effect resulting in excitation.