

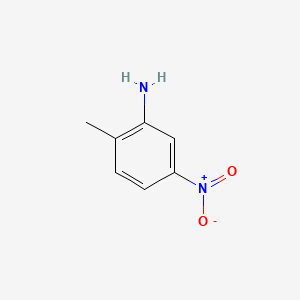

1. 5-nitro-2-toluidine
2. 5-nitro-2-toluidine Monohydrochloride
3. 5-nitro-2-toluidine Sulfate
4. 5-nitro-ortho-toluidine
1. 99-55-8
2. 2-amino-4-nitrotoluene
3. 5-nitro-o-toluidine
4. 2-methyl-5-nitrobenzenamine
5. Fast Scarlet G
6. Pnot
7. Scarlet G Base
8. 4-nitro-2-aminotoluene
9. Devol Scarlet B
10. 3-nitro-6-methylaniline
11. Fast Scarlet G Base
12. Benzenamine, 2-methyl-5-nitro-
13. 5-nitro-2-toluidine
14. Diabase Scarlet G
15. Scarlet Base Nsp
16. Fast Red Sg Base
17. O-toluidine, 5-nitro-
18. Fast Scarlet Base G
19. 6-methyl-3-nitroaniline
20. 1-amino-2-methyl-5-nitrobenzene
21. Fast Scarlet Base J
22. Fast Scarlet J Salt
23. Fast Scarlet T Base
24. Lake Scarlet G Base
25. Daito Scarlet Base G
26. Devol Scarlet G Salt
27. Diazo Fast Scarlet G
28. Azofix Scarlet G Salt
29. Fast Scarlet Gc Base
30. Scarlet Base Ciba Ii
31. Scarlet Base Irga Ii
32. Kayaku Scarlet G Base
33. Mitsui Scarlet G Base
34. Azogene Fast Scarlet G
35. Lithosol Orange R Base
36. Symulon Scarlet G Base
37. C.i. Azoic Diazo Component 12
38. Amarthol Fast Scarlet G Base
39. Hiltonil Fast Scarlet G Base
40. 2-methyl-5-nitro-benzeneamine
41. Naphthanil Scarlet G Base
42. Fast Scarlet G Salt
43. Naphtoelan Fast Scarlet G Base
44. Sugai Fast Scarlet G Base
45. Azoene Fast Scarlet Gc Base
46. Azoene Fast Scarlet Gc Salt
47. Amarthol Fast Scarlet G Salt
48. Dainichi Fast Scarlet G Base
49. Hiltonil Fast Scarlet G Salt
50. 5-nitro-2-methylaniline
51. Hiltonil Fast Scarlet Gc Base
52. Naphtoelan Fast Scarlet G Salt
53. 5-nitro-ortho-toluidine
54. Fast Scarlet M4nt Base
55. Rcra Waste Number U181
56. Azoic Diazo Component 12
57. Nci-c01843
58. C.i. 37105
59. Ci 37105
60. O-amino-p-nitrotoluene
61. 433myh2dwm
62. Azoic Diazo Component 12, Base
63. Dtxsid4020959
64. Chebi:66891
65. Nsc-8947
66. Mfcd00007741
67. Dtxcid60959
68. 2-methyl-5-nitro Aniline
69. Cas-99-55-8
70. Smr000343278
71. Ccris 483
72. Ci Azoic Diazo Component 12
73. Hsdb 4144
74. Fast Scarlet Mn4t Base
75. Nsc 8947
76. Einecs 202-765-8
77. Rcra Waste No. U181
78. Unii-433myh2dwm
79. 2-methyl-5-nitrophenylamine
80. Brn 0879021
81. Ai3-01557
82. Conazoic Diazo Ab
83. Amino-nitro-toluene
84. C.i. Azoic Diazo Component No. 12
85. 3-nitro-6-methylanilin
86. 2-methyl,5-nitroaniline
87. 2-amino-4-nitro Toluene
88. 2-amino-4-nitro-toluene
89. 2-methyl-5-nitro-aniline
90. 2-methyl-5-nitroaniline-
91. Fast Scarlet M 4nt Base
92. Ec 202-765-8
93. Wln: Zr B1 Enw
94. Schembl2313
95. Azoene Fast Scarlet G Salt
96. 4-12-00-01807 (beilstein Handbook Reference)
97. Mls000517118
98. Mls002152874
99. 2-methyl-5-nitro-phenylamine
100. (2-methyl-5-nitrophenyl)amine
101. Chembl324642
102. 2-methyl-5-nitroaniline, 95%
103. 2-methyl-5-nitroaniline, 98%
104. 4-nitro-2-amino-1-methylbenzene
105. Nsc8947
106. 1-methyl-2-amino-4-nitrobenzene
107. 2-methyl-5-nitroaniline, 98+%
108. Hms2679n16
109. Bcp24941
110. Cs-m3392
111. Str03005
112. 5-nitro-o-toluidine [hsdb]
113. Tox21_201945
114. Tox21_300134
115. Stl163886
116. Akos000119136
117. Sb10516
118. 5-nitro-ortho-toluidine [iarc]
119. 5-nitro-o-toluidine, Analytical Standard
120. Ncgc00091592-01
121. Ncgc00091592-02
122. Ncgc00091592-03
123. Ncgc00091592-04
124. Ncgc00254217-01
125. Ncgc00259494-01
126. Db-030868
127. 2-methyl-5-nitroaniline, Analytical Standard
128. Am20060769
129. Ft-0620718
130. Ft-0620719
131. N0115
132. En300-17407
133. D77508
134. Ah-034/32850060
135. Q-100917
136. 2-amino-4-nitrotoluene 10 Microg/ml In Acetonitrile
137. 2-amino-4-nitrotoluene 100 Microg/ml In Acetonitrile
138. Q27135494
139. Z56926522
140. F2190-0478
| Molecular Weight | 152.15 g/mol |
|---|---|
| Molecular Formula | C7H8N2O2 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 71.8 |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 155 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
5-Nitro-ortho-toluidine was identified as a product of the microbial transformation by Mucrosporium sp. of 2,4-dinitrotoluene, a compound common in water discharges and effluents from ammunition plants, ammunition loading facilities and sites for the destruction of stockpiled weapons ... . 5-Nitro-ortho-toluidine was identified as an intermediate in the anaerobic biotransformation of 2,4-dinitrotoluene added to a sample of municipal activated sludge ... . 5-Nitro-ortho-toluidine is formed enzymatically from 2,4-dinitrotoluene by liver and lung microsomal fractions from mice ... by liver microsomal and cytosolic fractions from rats ... and by intestinal microorganisms from rats and mice ... . 5-Nitro-ortho-toluidine and its N-acetyl derivative were identified as minor metabolites of 2,4-(14)C-dinitrotoluene when this compound was administered orally to male and female Fischer 344 rats at doses of 35, 63 or 100 mg/kg bw ... . Human intestinal microflora catalyse the reductive metabolism of 2,4-dinitrotoluene to 5-nitro-ortho-toluidine and 4-amino-2-nitrotoluene via nitroso intermediates ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. 48:171 (1991)