

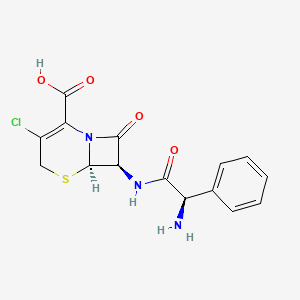

1. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-chloro-8-oxo-, (6r-(6alpha,7beta(r*)))-
2. Ceclor
3. Cefaclor Anhydrous
4. Cefaclor Monohydrate
5. Keclor
6. Lilly 99638
7. S 6472
8. S-6472
9. S6472
1. 53994-73-3
2. Cephaclor
3. Ceclor
4. Cefaclor Anhydrous
5. Cefaclorum
6. Kefral
7. Panoral
8. Raniclor
9. Cefaclor [inn]
10. Cefaclor Hydrate
11. Cefaclorum [inn-latin]
12. 3-chloro-7-d-(2-phenylglycinamido)-3-cephem-4-carboxylic Acid
13. 143059-69-2
14. S 6472
15. Cefaclor Impurity C
16. Chebi:3478
17. Ccl
18. 3z6fs3ik0k
19. Mls000069617
20. (6r,7r)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
21. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid,7-[[(2r)-aminophenylacetyl]amino]-3-chloro-8-oxo-, (6r,7r)-
22. 53994-73-3 (free)
23. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
24. (6r,7r)-7-{[(2r)-2-amino-2-phenylacetyl]amino}-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
25. Ncgc00022612-04
26. Alfacet
27. Smr000058250
28. Dsstox_cid_2748
29. Dsstox_rid_76713
30. Dsstox_gsid_22748
31. Dystaclor Mr
32. L-kefral
33. Ceclor Cd
34. Alenfral (tn)
35. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-chloro-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
36. (6r,7r)-7-[(2r)-2-amino-2-phenylacetamido]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
37. Cas-53994-73-3
38. Unii-3z6fs3ik0k
39. Cefaclorimpurityc
40. S-6472
41. Cefaclor,(s)
42. (6r,7r)-7-(((2r)-2-amino-2-phenylacetyl)amino)-3-chloro-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
43. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-2-amino-2-phenylacetyl)amino)-3-chloro-8-oxo-, (6r,7r)-
44. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-, (6r,7r)-
45. Einecs 258-909-5
46. Mfcd00151471
47. Cefaclor (jp17)
48. Spectrum_001070
49. Specplus_000947
50. Cefaclor [jan]
51. Cefaclor [mi]
52. Prestwick0_000485
53. Prestwick0_001102
54. Prestwick1_000485
55. Prestwick1_001102
56. Prestwick2_000485
57. Prestwick2_001102
58. Prestwick3_000485
59. Prestwick3_001102
60. Spectrum2_001189
61. Spectrum3_001858
62. Spectrum4_000093
63. Spectrum5_001727
64. Cefaclor [who-dd]
65. Chembl680
66. Epitope Id:117133
67. Schembl33540
68. Bspbio_000349
69. Bspbio_001204
70. Bspbio_003276
71. Kbiogr_000386
72. Kbioss_001550
73. Cid_51039
74. Mls001424193
75. Divk1c_007043
76. Spectrum1500771
77. Spbio_001237
78. Spbio_002270
79. Spbio_003082
80. Bpbio1_000385
81. Bpbio1_001326
82. Dtxsid3022748
83. Bdbm42131
84. Kbio1_001987
85. Kbio2_001550
86. Kbio2_004118
87. Kbio2_006686
88. Kbio3_002777
89. Bcpp000294
90. Hms1571m06
91. Hms1921g22
92. Hms2052c11
93. Hms2092k08
94. Hms2233m13
95. Pharmakon1600-01500771
96. (6r,7r)-7-[[(2s)-2-amino-1-oxo-2-phenylethyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
97. Hy-b0198
98. Zinc3812869
99. Tox21_110880
100. Ccg-40264
101. Nsc757422
102. Akos024282614
103. Tox21_110880_1
104. Bcp9000501
105. Cefaclor, Analytical Reference Material
106. Db00833
107. Nc00354
108. 7beta-{[(2r)-2-amino-2-phenylacetyl]amino}-3-chloro-3,4-didehydrocepham-4-carboxylic Acid
109. Ncgc00015260-22
110. Ncgc00022612-05
111. Ncgc00022612-06
112. Ncgc00022612-07
113. Ncgc00022612-10
114. Ncgc00022612-11
115. 7-((2r)-2-amino-2-phenylacetylamino)(7r,7ar)-3-chloro-6-oxo-2h,7h-azetidino[2, 1-b]1,3-thiazine-4-carboxylic Acid
116. As-74992
117. Sbi-0051606.p002
118. Ab00052174
119. C3478
120. C-2461
121. C06877
122. D00256
123. Ab00052174_15
124. Ab00052174_16
125. Cefaclor, Antibiotic For Culture Media Use Only
126. A900728
127. Q415167
128. Sr-05000001556
129. Sr-05000001556-1
130. Brd-k20338176-002-03-5
131. 7-(d-2-amino-2-phenylacetamido)-3-chloro-3-cephem-4-carboxylic
132. (6r,7r)-7-((s)-2-amino-2-phenylacetamido)-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
133. (6r,7r)-7-[(r)-2-amino-2-phenylacetamido]-3-chloro-8-oxo-5-thia-1-azabicyclo[4,2,0]oct-2-ene-2-carboxylic Acid
134. (6r,7r)-7-[[(2r)-2-amino-2-phenyl-acetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
135. (6r,7r)-7-[[(2r)-2-azaniumyl-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
136. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-chloro-8-oxo-, (6r-(6-alpha,7-beta(r*)))-
| Molecular Weight | 367.8 g/mol |
|---|---|
| Molecular Formula | C15H14ClN3O4S |
| XLogP3 | -1.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 367.0393548 g/mol |
| Monoisotopic Mass | 367.0393548 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cefaclor |
| PubMed Health | Cefaclor (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cefaclor is a semisynthetic cephalosporin antibiotic for oral administration. It is chemically designated as 3-chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate. The molecular formula for cefaclor is C15H14ClN3O4SH2O and the... |
| Active Ingredient | Cefaclor |
| Dosage Form | Tablet, extended release; Capsule; For suspension |
| Route | Oral |
| Strength | eq 375mg base/5ml; eq 250mg base/5ml; eq 500mg base; eq 187mg base/5ml; eq 250mg base; eq 125mg base/5ml; eq 375mg base |
| Market Status | Prescription |
| Company | Teva; Yung Shin Pharm; Hikma |
| 2 of 2 | |
|---|---|
| Drug Name | Cefaclor |
| PubMed Health | Cefaclor (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cefaclor is a semisynthetic cephalosporin antibiotic for oral administration. It is chemically designated as 3-chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate. The molecular formula for cefaclor is C15H14ClN3O4SH2O and the... |
| Active Ingredient | Cefaclor |
| Dosage Form | Tablet, extended release; Capsule; For suspension |
| Route | Oral |
| Strength | eq 375mg base/5ml; eq 250mg base/5ml; eq 500mg base; eq 187mg base/5ml; eq 250mg base; eq 125mg base/5ml; eq 375mg base |
| Market Status | Prescription |
| Company | Teva; Yung Shin Pharm; Hikma |
For the treatment of certain infections caused by bacteria such as pneumonia and ear, lung, skin, throat, and urinary tract infections.
FDA Label
Cefaclor is a second generation cephalosporin antibiotic with a spectrum resembling first-generation cephalosporins. In vitro tests demonstrate that the bactericidal action of the cephalosporins results from inhibition of cell-wall synthesis. As indicated by _in vitro_ and _in vivo_ clinical studies, cefaclor was shown to be effective against most strains of Gram positive aerobes - Staphylococci (including coagulase-positive, coagulase-negative, and penicillinase-producing strains), Streptococcus pneumoniae, Streptococcus pyogenes (group A ß-hemolytic streptococci), as well as Gram-negative aerobes - Escherichia coli, Haemophilus influenzae (including ß-lactamase-producing ampicillin-resistant strains), Klebsiella sp, and Proteus mirabilis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DC - Second-generation cephalosporins
J01DC04 - Cefaclor
Absorption
Well absorbed after oral administration, independent of food intake.
Route of Elimination
Approximately 60% to 85% of the drug is excreted unchanged in the urine within 8 hours, the greater portion being excreted within the first 2 hours.
No appreciable biotransformation in liver (approximately 60% to 85% of the drug is excreted unchanged in the urine within 8 hours).
0.6-0.9 hour
Cefaclor, like the penicillins, is a beta-lactam antibiotic. By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, it inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins. It is possible that cefaclor interferes with an autolysin inhibitor.