

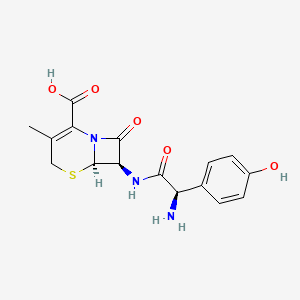

1. 4 Hydroxycephalexin
2. 4-hydroxycephalexin
3. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((amino(4-hydroxyphenyl)acetyl)amino)-3-methyl-8-oxo-, (6r-(6alpha,7beta(r*)))-
4. Bidocef
5. Bl S 578
6. Bl S578
7. Bl-s 578
8. Bl-s578
9. Bls 578
10. Bls578
11. Cefadroxil Anhydrous
12. Cefadroxil Monohydrate
13. Cephadroxyl
14. Duricef
15. S 578
16. S-578
17. S578
18. Ultracef
1. 50370-12-2
2. Cefadroxil Anhydrous
3. Cephadroxil
4. Cefadroxilo
5. Cefadroxilum
6. D-cefadroxil
7. Cefadroxil [inn]
8. Bl-s578
9. Anhydrous Cefadroxil
10. Chebi:3479
11. (6r,7r)-7-{[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
12. (6r,7r)-7-[[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
13. Bl-s 578
14. Cdx
15. Q525pa8jjb
16. Duracef
17. Nsc-756664
18. Dsstox_cid_2749
19. Dsstox_rid_76714
20. Dsstox_gsid_22749
21. Mjf-11567-3
22. (6r,7r)-7-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
23. Cefadroxil (as Monohydrate)
24. Cefadroxilum [inn-latin]
25. Cefadroxilo [inn-spanish]
26. (6r,7r)-7-[(2r)-2-amino-2-(4-hydroxyphenyl)acetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
27. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-amino(4-hydroxyphenyl)acetyl)amino)-3-methyl-8-oxo-, (6r,7r)-
28. Sumacef (tn)
29. Cefadroxil/cefadroxil Hemihydrate
30. S 578
31. S-578
32. Unii-q525pa8jjb
33. Cefradroxil
34. Cefadrops
35. Ncgc00016858-01
36. (6r,7r)-7-(((2r)-2-amino-2-(4-hydroxyphenyl)acetyl)amino)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
37. Einecs 256-555-6
38. Cas-50370-12-2
39. Cefadroxil, Anhydrous
40. Cefadroxil (jp17)
41. Spectrum_000104
42. Cefadroxil [mi]
43. Cefadroxil [jan]
44. (6r,7r)-7-[[(2r)-2-azaniumyl-2-(4-hydroxyphenyl)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
45. Prestwick0_000434
46. Prestwick1_000434
47. Prestwick2_000434
48. Prestwick3_000434
49. Spectrum2_000087
50. Spectrum4_000266
51. Spectrum5_000663
52. Epitope Id:117131
53. Cefadroxil [who-dd]
54. Chembl1644
55. Bspbio_000448
56. Kbiogr_000732
57. Kbioss_000544
58. (6r,7r)-7-((r)-2-amino-2-(p-hydroxyphenyl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
59. Mls002207219
60. Divk1c_000409
61. Schembl151320
62. Spbio_000014
63. Spbio_002387
64. Cefadroxil, Analytical Standard
65. Bpbio1_000494
66. Gtpl4831
67. Dtxsid8022749
68. Hms501e11
69. Kbio1_000409
70. Kbio2_000544
71. Kbio2_003112
72. Kbio2_005680
73. Ninds_000409
74. Hms1569g10
75. Hms2096g10
76. Hms3713g10
77. Pharmakon1600-01500163
78. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-2-amino-2-(4-hydroxyphenyl)acetyl)amino)-3-methyl-8-oxo-, (6r,7r)-
79. Hy-b1190
80. Zinc3830391
81. Tox21_110651
82. Bbl033696
83. Bdbm50350467
84. Mfcd00865091
85. Nsc756664
86. Stk801939
87. Akos005622555
88. Tox21_110651_1
89. Ccg-220434
90. Cs-4696
91. Db01140
92. Idi1_000409
93. Ncgc00179556-01
94. Ncgc00179556-03
95. As-11655
96. Smr001306770
97. Cefprozil Impurity B [ep Impurity]
98. Sbi-0051305.p003
99. Ab00513838
100. Cefprozil E Impurity B [ep Impurity]
101. C-2462
102. C06878
103. D00257
104. D81830
105. 592c878
106. Q2319020
107. Cefprozil Monohydrate Impurity B [ep Impurity]
108. Cefadroxil Monohydrate, Antibiotic For Culture Media Use Only
109. (7r)-7-((r)-2-amino-2-(4-hydroxyphenyl)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
110. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((amino(4-hydroxyphenyl)acetyl)amino)-3-methyl-8-oxo-, (6r-(6-alpha,7-beta(r*)))-
111. 7beta-{[(2r)-2-amino-2-(4-hydroxyphenyl)acetyl]amino}-3-methyl-3,4-didehydrocepham-4-carboxylic Acid
| Molecular Weight | 363.4 g/mol |
|---|---|
| Molecular Formula | C16H17N3O5S |
| XLogP3 | -2.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 363.08889182 g/mol |
| Monoisotopic Mass | 363.08889182 g/mol |
| Topological Polar Surface Area | 158 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 629 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cefadroxil |
| PubMed Health | Cefadroxil (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cefadroxil monohydrate, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is a white to yellowish-white crystalline powder. It is soluble in water and it is acid-stable. It is chemically designated as 5-Thia-1- azab... |
| Active Ingredient | Cefadroxil/cefadroxil hemihydrate |
| Dosage Form | Tablet; Capsule; For suspension |
| Route | Oral |
| Strength | eq 250mg base/5ml; eq 500mg base; eq 125mg base/5ml; eq 500mg base/5ml; eq 1gm base |
| Market Status | Prescription |
| Company | Ranbaxy; Teva Pharms; Aurobindo; Aurobindo Pharma; Lupin; Sandoz; Hikma Pharms; Orchid Hlthcare; Hikma |
| 2 of 2 | |
|---|---|
| Drug Name | Cefadroxil |
| PubMed Health | Cefadroxil (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cefadroxil monohydrate, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is a white to yellowish-white crystalline powder. It is soluble in water and it is acid-stable. It is chemically designated as 5-Thia-1- azab... |
| Active Ingredient | Cefadroxil/cefadroxil hemihydrate |
| Dosage Form | Tablet; Capsule; For suspension |
| Route | Oral |
| Strength | eq 250mg base/5ml; eq 500mg base; eq 125mg base/5ml; eq 500mg base/5ml; eq 1gm base |
| Market Status | Prescription |
| Company | Ranbaxy; Teva Pharms; Aurobindo; Aurobindo Pharma; Lupin; Sandoz; Hikma Pharms; Orchid Hlthcare; Hikma |
For the treatment of the following infections (skin, UTI, ENT) caused by; S. pneumoniae, H. influenzae, staphylococci, S. pyogenes (group A beta-hemolytic streptococci), E. coli, P. mirabilis, Klebsiella sp, coagulase-negative staphylococci and Streptococcus pyogenes
FDA Label
Cefadroxil, a first-generation cephalosporin antibiotic, is used to treat urinary tract infections, skin and skin structure infections, pharyngitis, and tonsillitis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DB05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DB - First-generation cephalosporins
J01DB05 - Cefadroxil
Absorption
Cefadroxil is well absorbed on oral administration; food does not interfere with its absorption.
Route of Elimination
Over 90% of the drug is excreted unchanged in the urine within 24 hours. Cefadroxil was detected in the placenta and breast milk.
1.5 hours
Like all beta-lactam antibiotics, cefadroxil binds to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, causing the inhibition of the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that cefadroxil interferes with an autolysin inhibitor.