


1. Axpim
2. Bmy 28142
3. Bmy-28142
4. Bmy28142
5. Cefepim
6. Cefepime Hydrochloride
7. Maxipime
8. Quadrocef
1. Maxipime
2. 88040-23-7
3. Cefepima
4. Cefepimum [latin]
5. Cefepima [spanish]
6. Cefepimum
7. Bmy-28142
8. Cfpm
9. Axepim
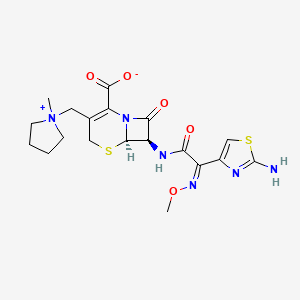
10. (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(1-methylpyrrolidin-1-ium-1-yl)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
11. 807pw4vqe3
12. 1-(((6r,7r)-7-(2-(2-amino-4-thiazolyl)glyoxylamido)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-1-methylpyrrolidinium Hydroxide, Inner Salt, 7(sup 2)-(z)-(o-methyloxime)
13. Vnrx-5022
14. Chebi:478164
15. J01de01
16. Bmy 28142
17. (6r,7r)-7-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-[(1-methylpyrrolidinium-1-yl)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
18. Cefepime (usan/inn)
19. Unii-807pw4vqe3
20. Cefepime [usan:inn:ban]
21. Cefepime, Antibiotic For Culture Media Use Only
22. Cefepime [usan]
23. Cefepime [inn]
24. Cefepime [mi]
25. Cefepime [vandf]
26. Cefepime [who-dd]
27. Chembl186
28. Schembl65720
29. Pyrrolidinium, 1-((7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-1-methyl-, Hydroxide, Inner Salt, (6r-(6alpha,7beta(z)))-
30. Dtxsid70873208
31. Bcpp000292
32. Hms2089m18
33. Hy-b0692
34. Bdbm50350470
35. Akos015850865
36. Akos016014147
37. Bcp9000504
38. Db01413
39. 7beta-[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido]-3-[(1-methylpyrrolidinium-1-yl)methyl]-3,4-didehydrocepham-4-carboxylate
40. As-76125
41. Cs-0009590
42. C-2468
43. C08111
44. D02376
45. Ab01275446-01
46. Q27294816
47. 1-{[(6r,7r)-7-[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido]-2-carboxylato-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl}-1-methylpyrrolidin-1-ium
48. Pyrrolidinium, 1-((7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-1-methyl-, Hydroxide, Inner Salt, (6r-(6.alpha.,7.beta.(z)))-
| Molecular Weight | 480.6 g/mol |
|---|---|
| Molecular Formula | C19H24N6O5S2 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 6 |
| Exact Mass | 480.12496023 g/mol |
| Monoisotopic Mass | 480.12496023 g/mol |
| Topological Polar Surface Area | 204 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 869 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Maxipime |
| PubMed Health | Cefepime (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | MAXIPIME (cefepime hydrochloride, USP) is a semi-synthetic, broad spectrum, cephalosporin antibiotic for parenteral administration. The chemical name is 1-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)-glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0] o... |
| Active Ingredient | Cefepime hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Hospira |
| 2 of 2 | |
|---|---|
| Drug Name | Maxipime |
| PubMed Health | Cefepime (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | MAXIPIME (cefepime hydrochloride, USP) is a semi-synthetic, broad spectrum, cephalosporin antibiotic for parenteral administration. The chemical name is 1-[[(6R,7R)-7-[2-(2-amino-4-thiazolyl)-glyoxylamido]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0] o... |
| Active Ingredient | Cefepime hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 500mg base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Hospira |
For the treatment of pneumonia (moderate to severe) caused by Streptococcus pneumoniae, including cases associated with concurrent bacteremia, Pseudomonas aeruginosa, Klebsiella pneumoniae, or Enterobacter species. Also for empiric treatment of febrile neutropenic patients and uncomplicated and complicated urinary tract infections (including pyelonephritis) caused by Escherichia coli or Klebsiella pneumoniae, when the infection is severe, or caused by Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis, when the infection is mild to moderate, including cases associated with concurrent bacteremia with these microorganisms. Also for the treatment of uncomplicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible strains only) or Streptococcus pyogenes and complicated intra-abdominal infections (used in combination with metronidazole) caused by Escherichia coli, viridans group streptococci, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter species, or Bacteroides fragilis.
FDA Label
Cefepime is a fourth-generation cephalosporin antibiotic developed in 1994. Cefepime is active against Gram-positive and Gram-negative bacteria, with greater activity against both than third-generation antibiotics. Cefepime has good activity against important pathogens including Pseudomonas aeruginosa, Staphylococcus aureus, Enterobacteriaceae, and multiple drug resistant Streptococcus pneumoniae. Whereas other cephalosporins are degraded by many plasmid- and chromosome-mediated beta-lactamases, cefepime is stable and is a front line agent when infection with Enterobacteriaceae is known or suspected
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DE - Fourth-generation cephalosporins
J01DE01 - Cefepime
Absorption
The absolute bioavailability of cefepime after an IM dose of 50 mg/kg was 82.3 (±15)% in eight patients.
Route of Elimination
Elimination of cefepime is principally via renal excretion with an average (SD) half-life of 2 (0.3) hours and total body clearance of 120 (8) mL/min in healthy volunteers. Cefepime is excreted in human milk.
Volume of Distribution
18.0 2.0 L
0.3 0.1 L/kg [Pediatric]
Clearance
120 mL/min [Healthy adult male receiving a single 30-minute IV infusions of cefepime]
3.3 +/-1.0 mL/min/kg [Petriatic patients (2 months 11 years of age) receiving a single IV dose]
Hepatic. Cefepime is metabolized to N-methylpyrrolidine (NMP) which is rapidly converted to the N-oxide (NMP-N-oxide).
2.0 (± 0.3) hours in normal patients. The average half-life in patients requiring hemodialysis was 13.5 (± 2.7) hours and in patients requiring continuous peritoneal dialysis was 19.0 (± 2.0) hours.
Cephalosporins are bactericidal and have the same mode of action as other beta-lactam antibiotics (such as penicillins). Cephalosporins disrupt the synthesis of the peptidoglycan layer of bacterial cell walls. The peptidoglycan layer is important for cell wall structural integrity, especially in Gram-positive organisms. The final transpeptidation step in the synthesis of the peptidoglycan is facilitated by transpeptidases known as penicillin binding proteins (PBPs).