


1. S-649266
1. 1225208-94-5
2. Cefiderocol [inn]
3. Gsk2696266
4. Cefiderocol [who-dd]
5. Sz34omg6e8
6. S-649266
7. Cefiderocol (usan)
8. Cefiderocol [usan]
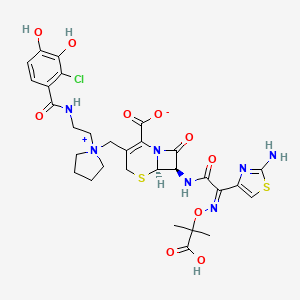
9. (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetyl]amino]-3-[[1-[2-[(2-chloro-3,4-dihydroxybenzoyl)amino]ethyl]pyrrolidin-1-ium-1-yl]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
10. Pyrrolidinium, 1-(((6r,7r)-7-(((2z)-2-(2-amino-4-thiazolyl)-2-((1-carboxy-1-methylethoxy)imino)acetyl)amino)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3-yl)methyl)-1-(2-((2-chloro-3,4-dihydroxybenzoyl)amino)ethyl)-, Inner Salt
11. Rsc 649266
12. Pyrrolidinium, 1-[[(6r,7r)-7-[[(2z)-2-(2-amino-4-thiazolyl)-2-[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-[2-[(2-chloro-3,4-dihydroxybenzoyl)amino]ethyl]-, Inner Salt
13. Cefiderocol [mi]
14. Cefiderocol [usan:inn]
15. Unii-sz34omg6e8
16. Chembl3989974
17. Schembl22508010
18. Dtxsid401098052
19. Akos037648584
20. Db14879
21. Gsk 2696266
22. Bs-14716
23. Hy-17628
24. Cs-0016784
25. D11302
26. S 649266
27. (6r,7r)-7-((2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(((2-carboxypropan-2-yl)oxy)imino)acetamido)-3-((1-(2-(2-chloro-3,4-dihydroxybenzamido)ethyl)pyrrolidin-1-ium-1-yl)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate
| Molecular Weight | 752.2 g/mol |
|---|---|
| Molecular Formula | C30H34ClN7O10S2 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 12 |
| Exact Mass | 751.1497103 g/mol |
| Monoisotopic Mass | 751.1497103 g/mol |
| Topological Polar Surface Area | 310 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 1440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cefiderocol is indicated for the treatment of complicated urinary tract infections with or without pyelonephritis.
FDA Label
Fetcroja is indicated for the treatment of infections due to aerobic Gram-negative organisms in adults with limited treatment options (see sections 4. 2, 4. 4 and 5. 1).
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Treatment of infections due to aerobic Gram-negative bacteria
Similarly to other cephalosporins, cefiderocol exerts bactericidal activity against a range of bacterial species. Cefiderocol has primarily shown efficacy against aerobic Gram negative bacteria including *Escherichia coli*, *Klebsiella pneumoniae*, and *Pseudomonas aeruginosa*.
J01D
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DI - Other cephalosporins and penems
J01DI04 - Cefiderocol
Absorption
A single intravenous dose of 2 g of cefiderocol in healthy patients produces a Cmax of 89.7 mg/L and an AUC of 386 mg\*h/L. In patients with complicated urinary tract infections and a creatinine clearance of at least 60 mL/min, doses of 2 g cefiderocol every 8 hours produced an AUC of 394.7 mg*h/L and a Cmax of 138 mg/L. However the infusion rate for this chronic dosing was 3 times the recommended rate. Cmax and AUC are known to increase proportionally with dosage.
Route of Elimination
98.6% of cefiderocol is eliminated in the urine with 90.6% as the unchanged parent drug. The remaining 8% is eliminated as metabolites. 2.8% is eliminated in the feces. Less than 10% of cefiderocol is metabolized.
Volume of Distribution
Cefiderocol has a mean volume of distribution of 18 L.
Clearance
Cefiderocol has a mean clearance of 5.18 L/h.
Cefiderocol undergoes a small degree of metabolism to a cefiderocol epimer at the 7 position, cefiderocol catechol-3-methoxy and -4-methoxy, and a pyrrolidine chlorobenzamide product (PCBA). PCBA undergoes further metabolism to sulfated, methylated, and glucuronidated metabolites. The enzymes involved in these reactions have yet to be identified and cefiderocol has not been shown to interfere in the metabolism of other agents.
The terminal elimination half-life of cefiderocol is 2-3 h.
Cefiderocol acts by binding to and inhibiting penicillin-binding proteins (PBPs), preventing cell wall synthesis and ultimately causing death of the bacterial cell. Like other -lactam antibiotics cefiderocol is able to enter bacterial cells via passive diffusion through porins. Unlike other -lactams, cefiderocol contains a chlorocatechol group which allows it to chelate iron. Once bound to ferric iron cefiderocol is able to undergo active transport into bacterial cells through iron channels in the outer cell membrane such as those encoded by the *cirA* and *fiu* genes in *E. coli* or the *PiuA* gene in *P. aeruginosa*. Once inside the cell, cefiderocol binds to and inhibits PBP3 with high affinity thereby preventing the linking of peptodoglycan layers via the pentapeptide bridge. PBP1a, 1b, 2,and 4 are also bound and inhibited by cefiderocol but with a lesser potency than PBP3 and are therefore expected to contribute less to its antibacterial effect.