


1. Cefoxitin Sodium
2. Mfoxin
3. Mefoxin
4. Mefoxitin
5. Mk 306
6. Mk-306
7. Mk306
8. Sodium, Cefoxitin
1. 35607-66-0
2. Mefoxin
3. Cefoxitinum
4. Rephoxitin
5. Cephoxitin
6. Cefoxitina
7. Cefoxitine
8. Mefoxitin
9. Ceftoxitin
10. Cfx
11. Cenomycin
12. Chebi:209807
13. Chembl996
14. 6oev9dx57y
15. J01dc01
16. Dsstox_cid_2764
17. Dsstox_rid_76721
18. Dsstox_gsid_22764
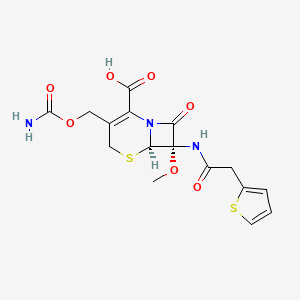
19. (6r,7s)-3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
20. (6r,7s)-3-(hydroxymethyl)-7-methoxy-8-oxo-7-(2-(2-thienyl)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid Carbamate (ester)
21. (6r,7s)-3-[(carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[2-(thiophen-2-yl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
22. (6r,7s)-3-((carbamoyloxy)methyl)-7-methoxy-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
23. Cas-35607-66-0
24. Ncgc00183034-01
25. Cefoxitine [inn-french]
26. Cefoxitinum [inn-latin]
27. Cefoxitina [inn-spanish]
28. Unii-6oev9dx57y
29. Cefoxotin
30. Cefoxitin [usan:inn:ban]
31. 4kow
32. Mk306
33. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-methoxy-8-oxo-7-((2-thienylacetyl)amino)-, (6r,7s)-
34. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 3-[[(aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-, (6r,7s)-
35. Einecs 252-641-2
36. Mfcd00072014
37. Spectrum_001399
38. Cefoxitin [inn]
39. Cefoxitin [mi]
40. Cefoxitin (usan/inn)
41. Cefoxitin [usan]
42. Prestwick0_000832
43. Prestwick1_000832
44. Prestwick2_000832
45. Prestwick3_000832
46. Spectrum2_000676
47. Spectrum3_001491
48. Spectrum4_000153
49. Spectrum5_001145
50. Cefoxitin [vandf]
51. Epitope Id:116201
52. C06887
53. Cefoxitin [usp-rs]
54. Cefoxitin [who-dd]
55. Schembl15971
56. Bspbio_000783
57. Bspbio_003101
58. Kbiogr_000626
59. Kbioss_001879
60. Divk1c_000576
61. Spbio_000771
62. Spbio_002704
63. Bpbio1_000863
64. Cefoxitin [orange Book]
65. Dtxsid1022764
66. Gtpl10937
67. Kbio1_000576
68. Kbio2_001879
69. Kbio2_004447
70. Kbio2_007015
71. Kbio3_002601
72. Ninds_000576
73. Bcp09061
74. Hy-b1825
75. Zinc3830449
76. Tox21_113009
77. Bdbm50335563
78. S5951
79. Akos022185232
80. Tox21_113009_1
81. Db01331
82. Ds-5384
83. Idi1_000576
84. Ncgc00093351-06
85. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-methoxy-8-oxo-7-((2-thienylacetyl)amino)-, (6r-cis)-
86. 73356-24-8
87. Sbi-0051927.p002
88. C3598
89. Cs-0013876
90. (6r,7r)-3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
91. (6r,7s)-3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
92. (6r,7s)-3-carbamoyloxymethyl-7-methoxy-8-oxo-7-(2-thiophen-2-yl-acetylamino)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
93. (6r,7s)-4-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
94. (6s,7r)-3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
95. (6s,7r)-4-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
96. 3-carbamoyloxymethyl-7-methoxy-8-oxo-7-(2-thiophen-2-yl-acetylamino)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
97. 3-carbamoyloxymethyl-7-methoxy-8-oxo-7-(2-thiophen-2-yl-acetylamino)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid Anion
98. 3-carbamoyloxymethyl-7-methoxy-8-oxo-7-(2-thiophen-3-yl-acetylamino)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid Anion
99. D02345
100. 607c660
101. A822898
102. Q2353907
103. (6r,7s)-3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-(2-(thiophen-2-yl)acetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
104. (6r,7s)-3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-[[2-(2-thienyl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
105. (6r,7s)-3-(carbamoyloxymethyl)-7-methoxy-8-oxo-7-[[2-(2-thienyl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid;cefoxitin
106. (6r,7s)-3-[(carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
107. (6r,7s)-3-[(carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[(thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
108. (6r,7s)-3-[[(aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- Carboxylic Acid
109. (6r,7s)-3-{[(aminocarbonyl)oxy]methyl}-7-(methyloxy)-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
110. (6r,7s)-4-[(carbamoyloxy)methyl]-7-methoxy-8-oxo-7-[(thiophen-2-enyl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
111. 3-[(carbamoyloxy)methyl]-7alpha-methoxy-7beta-[(thiophen-2-yl)acetamido]-3,4-didehydrocepham-4-carboxylic Acid
112. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid,3-[[(aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-, (6r-cis)-
113. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid,3-[[(aminocarbonyl)oxy]methyl]-7-methoxy-8-oxo-7-[(2-thienylacetyl)amino]-,(6r,7s)-
| Molecular Weight | 427.5 g/mol |
|---|---|
| Molecular Formula | C16H17N3O7S2 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 427.05079224 g/mol |
| Monoisotopic Mass | 427.05079224 g/mol |
| Topological Polar Surface Area | 202 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 744 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cefoxitin |
| PubMed Health | Cefoxitin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | Cefoxitin for Injection, USP contains cefoxitin sodium a semi-synthetic, broad-spectrum cephalosporin antibiotic for parenteral administration. It is derived from cephalosporin C, which is produced by Cephalosporium Acremonium. Its chemical name is s... |
| Active Ingredient | Cefoxitin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Acs Dobfar; Hospira; Hikma Maple; Hikma Farmaceutica; Antibioticos Brasil |
| 2 of 2 | |
|---|---|
| Drug Name | Cefoxitin |
| PubMed Health | Cefoxitin (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | Cefoxitin for Injection, USP contains cefoxitin sodium a semi-synthetic, broad-spectrum cephalosporin antibiotic for parenteral administration. It is derived from cephalosporin C, which is produced by Cephalosporium Acremonium. Its chemical name is s... |
| Active Ingredient | Cefoxitin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Acs Dobfar; Hospira; Hikma Maple; Hikma Farmaceutica; Antibioticos Brasil |
For the treatment of serious infections caused by susceptible strains microorganisms.
FDA Label
Cefoxitin is a cephamycin antibiotic often grouped with the second-generation cephalosporins. It is active against a broad range of gram-negative bacteria including anaerobes. The methoxy group in the 7a position provides cefoxitin with a high degree of stability in the presence of beta-lactamases, both penicillinases and cephalosporinases, of gram-negative bacteria.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DC - Second-generation cephalosporins
J01DC01 - Cefoxitin
Route of Elimination
Approximately 85 percent of cefoxitin is excreted unchanged by the kidneys over a 6-hour period, resulting in high urinary concentrations. Cefoxitin passes into pleural and joint fluids and is detectable in antibacterial concentrations in bile.
Minimal (approximately 85 percent of cefoxitin is excreted unchanged by the kidneys over a 6-hour period).
The half-life after an intravenous dose is 41 to 59 minutes.
The bactericidal action of cefoxitin results from inhibition of cell wall synthesis.