


1. Cefpiramide Sodium
2. Cefpiramide, Sodium Salt
3. Sm 1652
4. Sm-1652
5. Sumcefal
1. 70797-11-4
2. Cefpiramide Acid
3. Cefpiramido
4. Cefpiramidum
5. Wy-44635
6. Wy-44,635
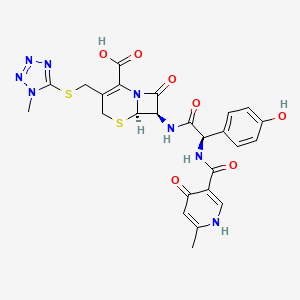
7. (6r,7r)-7-[[(2r)-2-(4-hydroxyphenyl)-2-[(6-methyl-4-oxo-1h-pyridine-3-carbonyl)amino]acetyl]amino]-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
8. Chebi:59213
9. P936ya152n
10. Nsc-759869
11. Ncgc00167444-01
12. Sm 1652
13. Sm-1652
14. (7r)-7-[[(2r)-2-(4-hydroxyphenyl)-2-[(6-methyl-4-oxo-1h-pyridine-3-carbonyl)amino]acetyl]amino]-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
15. (6r,7r)-7-((r)-2-(4-hydroxy-6-methylnicotinamido)-2-(4-hydroxyphenyl)acetamido)-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
16. Wy 44,635
17. Cefpiramidum [inn-latin]
18. Cefpiramido [inn-spanish]
19. Unii-p936ya152n
20. Cefpiramide [usan:usp:inn]
21. Wy 44635
22. Cefpiramide (usp/inn)
23. Cefpiramide [mi]
24. Cefpiramide [inn]
25. Cefpiramide [usan]
26. Cefpiramide [mart.]
27. Dsstox_cid_26630
28. Dsstox_rid_81778
29. Dsstox_gsid_46630
30. Schembl49253
31. Cefpiramide [usp-rs]
32. Cefpiramide [who-dd]
33. Chembl1201204
34. Dtxsid6046630
35. Gtpl12027
36. Cefpiramide [usp Impurity]
37. Hms3715h12
38. Hy-b1354
39. Zinc4215257
40. Tox21_112448
41. Mfcd00864893
42. S5186
43. Akos025311237
44. Ccg-221235
45. Db00430
46. Nsc 759869
47. Ncgc00167444-04
48. (6r,7r)-7-((r)-2-(4-hydroxy-6-methylnicotinamido)-2-(p-hydroxyphenyl)acetamido)-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
49. As-14170
50. Cas-70797-11-4
51. Cs-0013097
52. D03428
53. 797c114
54. Q4921174
55. Brd-k92872987-001-02-9
56. Cefpiramide Acid;cefpiramido;cefpiramidum;wy-44635;sm-1652
57. (6r,7r)-7-((r)-2-(4-hydroxy-6-methylnicotinamido)-2-(4-hydroxyphenyl)acetamido)-3-((1-methyl-1h-tetrazol-5-ylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
58. (6r,7r)-7-[(2r)-2-[(4-hydroxy-6-methylpyridin-3-yl)formamido]-2-(4-hydroxyphenyl)acetamido]-3-{[(1-methyl-1h-1,2,3,4-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
59. (6r,7r)-7-{[(2r)-2-{[(4-hydroxy-6-methylpyridin-3-yl)carbonyl]amino}-2-(4-hydroxyphenyl)acetyl]amino}-3-{[(1-methyl-1h-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
60. (7r)-7-((r)-2-(4-hydroxy-6-methylnicotinamido)-2-(4-hydroxyphenyl)acetamido)-3-((1-methyl-1h-tetrazol-5-ylthio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
61. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((((4-hydroxy-6-methyl-3-pyridinyl)carbonyl)amino)(4-hydroxyphenyl)acetyl)amino)-3-(((1-methyl-1h-tetrazol-5-yl)thio)methyl)-8-oxo-, (6r-(6alpha,7beta(r*)))-
62. 7beta-[(2r)-2-{[(4-hydroxy-6-methylpyridin-3-yl)carbonyl]amino}-2-(4-hydroxyphenyl)acetamido]-3-{[(1-methyl-1h-tetrazol-5-yl)sulfanyl]methyl}-3,4-didehydrocepham-4-carboxylic Acid
| Molecular Weight | 612.6 g/mol |
|---|---|
| Molecular Formula | C25H24N8O7S2 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 9 |
| Exact Mass | 612.12093748 g/mol |
| Monoisotopic Mass | 612.12093748 g/mol |
| Topological Polar Surface Area | 259 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For treatment of severe infections caused by susceptible bacteria such as P. aeruginosa.
Cefpiramide is a cephalosporin active against Pseudomonas aeruginosa. It has a broad spectrum of antibacterial activity. Cefpiramide works by inhibiting bacterial cell wall biosynthesis. The plasma half-lives of cefpiramide in rabbits, dogs, and rhesus monkeys were much longer than those of cefoperazone and cefazolin.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DD - Third-generation cephalosporins
J01DD11 - Cefpiramide
Absorption
Rapidly absorbed following intramuscular injection.
4.44 hours
The bactericidal activity of cefpiramide results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs).