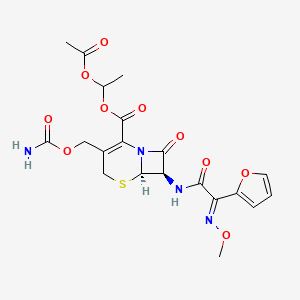

1. Acetoxyethylcefuroxime
2. Cci 15641
3. Cci-15641
4. Ceftin
5. Cefurax
6. Cefuroxime 1-acetoxyethyl Ester
7. Cepazine
8. Cetoxil
9. Curocef
10. Elobact
11. Novador
12. Novocef
13. Oraxim
14. Zinat
15. Zinnat
1. 97232-96-7
2. Cefuroxime Axetil, (e)-
3. 64544-07-6
4. (+/-)-cefuroxime Axetil, (e)-
5. Xsl6i3sb26
6. Ceftin
7. Cefuroxime Axetil E-isomers
8. 1-acetyloxyethyl (6r,7r)-3-(carbamoyloxymethyl)-7-[[(2e)-2-(furan-2-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
9. (1rs)-1-(acetyloxy)ethyl (6r,7r)-3-((carbamoyloxy)methyl)-7-(((e)-2-(furan-2-yl)-2-(methoxyimino)acetyl)amino)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2carboxylate
10. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-(((2e)-2-(2-furanyl)-2-(methoxyimino)acetyl)amino)-8-oxo-, 1-(acetyloxy)ethyl Ester, (6r,7r)-
11. Unii-xsl6i3sb26
12. Cefuroxime Axetil Impurity B [ep]
13. Cefuroximeaxetil
14. Cefuroxime Axetil Specified Impurity B [ep]
15. Schembl41099
16. Schembl379336
17. Dtxsid20242788
18. Mfcd00864991
19. Akos037643328
20. Ncgc00185751-06
21. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-((2-furanyl(methoxyimino)acetyl)amino)-8-oxo-, 1-(acetyloxy)ethyl Ester, (6r-(6alpha,7beta(e)))-
22. Cefuroxime Axetil E-isomers [usp-rs]
23. Cefuroxime Axetil Impurity B [ep Impurity]
24. Q3604308
25. 1-(acetyloxy)ethyl (6r,7r)-3-{[(aminocarbonyl)oxy]methyl}-7-({(2e)-2-furan-2-yl-2-[(methyloxy)imino]acetyl}amino)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
26. 1-acetoxyethyl (6r,7r)-3-carbamoyloxymethyl-7-[(z)-2-(fur-2-yl)-2-methoxyiminoacetamido]ceph-3-em-4-carboxylate
27. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(((aminocarbonyl)oxy)methyl)-7-((2-furanyl(methoxyimino)acetyl)amino)-8-oxo-, 1-(acetyloxy)ethyl Ester, (6r-(6.alpha.,7.beta.(e)))-
28. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 3-[[(aminocarbonyl)oxy]methyl]-7-[[(2z)-2-(2-furanyl)-2-(methoxyimino)acetyl]amino]-8-oxo-, 1- (acetyloxy)ethyl Ester, (6r,7r)-
| Molecular Weight | 510.5 g/mol |
|---|---|
| Molecular Formula | C20H22N4O10S |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 12 |
| Exact Mass | 510.10566408 g/mol |
| Monoisotopic Mass | 510.10566408 g/mol |
| Topological Polar Surface Area | 214 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 968 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cefuroxime axetil |
| Drug Label | Cefuroxime axetil tablets USP contain cefuroxime as cefuroxime axetil. Cefuroxime axetil is a semisynthetic, broad-spectrum cephalosporin antibiotic for oral administration.Chemically, cefuroxime axetil, the 1-(acetyloxy) ethyl ester of cefuroxime, i... |
| Active Ingredient | Cefuroxime axetil |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | eq 250mg base/5ml; eq 500mg base; eq 125mg base; eq 250mg base; eq 125mg base/5ml |
| Market Status | Prescription |
| Company | Wockhardt; Ranbaxy; Teva; Apotex; Alkem Labs; Aurobindo Pharma; Lupin; Orchid Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Cefuroxime axetil |
| Drug Label | Cefuroxime axetil tablets USP contain cefuroxime as cefuroxime axetil. Cefuroxime axetil is a semisynthetic, broad-spectrum cephalosporin antibiotic for oral administration.Chemically, cefuroxime axetil, the 1-(acetyloxy) ethyl ester of cefuroxime, i... |
| Active Ingredient | Cefuroxime axetil |
| Dosage Form | Tablet; For suspension |
| Route | Oral |
| Strength | eq 250mg base/5ml; eq 500mg base; eq 125mg base; eq 250mg base; eq 125mg base/5ml |
| Market Status | Prescription |
| Company | Wockhardt; Ranbaxy; Teva; Apotex; Alkem Labs; Aurobindo Pharma; Lupin; Orchid Hlthcare |
Anti-Bacterial Agent
National Library of Medicine's Medical Subject Headings. Cefuroxime axetil. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Ceftin tablets and oral suspension /are indicated for/ acute bacterial otitis media caused by Streptococcus pneumoniae, Haemophilusinfluenzae(including beta-lactamase-producing strains), Moraxellacatarrhalis (including beta-lactamase-producing strains), or Streptococcus pyogenes. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ICEFTIN (cefuroxime axetil) powder, for suspension CEFTIN (cefuroxime axetil) tablet, film coated (July 2011). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2f439b86-59d6-4350-aeb4-c904b3781db4
Ceftin tablets and oral suspension /are indicated for/ pharyngitis/tonsillitis caused by Streptococcus pyogenes. NOTE: The usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever, is penicillin given by the intramuscular route. Ceftin Tablets are generally effective in the eradication of streptococci from the nasopharynx; however, substantial data establishing the efficacy of cefuroxime in the subsequent prevention of rheumatic fever are not available. Please also note that in all clinical trials, all isolates had to be sensitive to both penicillin and cefuroxime. There are no data from adequate and well-controlled trials to demonstrate the effectiveness of cefuroxime in the treatment of penicillin-resistant strains of Streptococcus pyogenes. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ICEFTIN (cefuroxime axetil) powder, for suspension CEFTIN (cefuroxime axetil) tablet, film coated (July 2011). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2f439b86-59d6-4350-aeb4-c904b3781db4
Ceftin tablets /are indicated for/ acute bacterial maxillary sinusitis caused by Streptococcus pneumoniaeorHaemophilusinfluenzae (non-beta-lactamase-producing strains only). NOTE: In view of the insufficient numbers of isolates of beta-lactamase-producing strains of Haemophilusinfluenzae and Moraxellacatarrhalis that were obtained from clinical trials with Ceftin Tablets for patients with acute bacterial maxillary sinusitis, it was not possible to adequately evaluate the effectiveness of Ceftin Tablets for sinus infections known, suspected, or considered potentially to be caused by beta-lactamase-producing Haemophilusinfluenzae or Moraxellacatarrhalis. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ICEFTIN (cefuroxime axetil) powder, for suspension CEFTIN (cefuroxime axetil) tablet, film coated (July 2011). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2f439b86-59d6-4350-aeb4-c904b3781db4
For more Therapeutic Uses (Complete) data for Cefuroxime axetil (15 total), please visit the HSDB record page.
Patients should be advised that antibacterials (including cefuroxime) should only be used to treat bacterial infections and not used to treat viral infections (e.g., the common cold). Patients also should be advised about the importance of completing the full course of therapy, even if feeling better after a few days, and that skipping doses or not completing therapy may decrease effectiveness and increase the likelihood that bacteria will develop resistance and will not be treatable with cefuroxime or other antibacterials in the future.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 71
To reduce development of drug-resistant bacteria and maintain effectiveness of cefuroxime and other antibacterials, the drug should be used only for the treatment or prevention of infections proven or strongly suspected to be caused by susceptible bacteria. When selecting or modifying anti-infective therapy, use results of culture and in vitro susceptibility testing. In the absence of such data, consider local epidemiology and susceptibility patterns when selecting anti-infectives for empiric therapy.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 71
Prior to initiation of cefuroxime therapy, careful inquiry should be made concerning previous hypersensitivity reactions to cephalosporins, penicillins, or other drugs. Cefuroxime axetil /is/ contraindicated in patients who are hypersensitive to cefuroxime or other cephalosporins and should be used with caution in patients with a history of hypersensitivity to penicillins. Use of cephalosporins should be avoided in patients who have had an immediate-type (anaphylactic) hypersensitivity reaction to penicillins.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 71
Prescribing Ceftin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
US Natl Inst Health; DailyMed. Current Medication Information for ICEFTIN (cefuroxime axetil) powder, for suspension CEFTIN (cefuroxime axetil) tablet, film coated (July 2011). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2f439b86-59d6-4350-aeb4-c904b3781db4
For more Drug Warnings (Complete) data for Cefuroxime axetil (18 total), please visit the HSDB record page.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Following oral administration of cefuroxime axetil in pediatric patients with acute otitis media with effusion or with chronic or recurrent otitis media with effusion, cefuroxime is distributed into middle ear effusions. In a study in pediatric patients 1-4 years of age with acute otitis media with effusion who received a single 15 mg/kg dose of cefuroxime as cefuroxime axetil oral suspension, cefuroxime concentrations in middle ear effusions 2-5 hours after a dose ranged from 0.2-3.6 ug/mL; concurrent serum concentrations were 2.8-7.3 ug/mL.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 74
In pharmacokinetic studies of cefuroxime axetil oral suspension in children, the drug was administered postprandially or with food; no data are available regarding absorption of the suspension in fasting children. Following oral administration to children 3 months to 12 years of age (mean age: 23 months) of a single 10-, 15-, or 20-mg/kg dose of commercially available cefuroxime axetil oral suspension concomitantly with milk or milk products, peak serum cefuroxime concentrations are attained approximately 3.6, 2.7, or 3.1 hours after the dose, respectively, and average 3.3, 5.1, or 7 mcg/mL, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 73
Results of a study in healthy adults indicate that cefuroxime axetil oral suspensions containing 125 mg/5 mL or 250 mg/5 mL are bioequivalent. In healthy adults who received a 250-mg dose of cefuroxime axetil given as a suspension containing 125 mg/5 mL or 250 mg/5 mL with food, peak plasma concentrations of cefuroxime were 2.4 or 2.2 aug/mL, respectively, and were attained 3 hours after the dose.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 73
Following oral administration in adults of a single 125-mg, 250-mg, 500-mg, or 1-g dose of commercially available cefuroxime axetil tablets immediately following a meal, peak serum cefuroxime concentrations are attained approximately 2-3 hours after the dose and average 2.1, 4.1, 7, or 13.6 ug/mL, respectively; serum concentrations 6 hours after the dose average 0.3, 0.7, 2.2, or 3.4 ug/mL, respectively. AUC of the drug in these individuals averaged 6.7, 12.9, 27.4, or 50 mcg-h/mL, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 73
For more Absorption, Distribution and Excretion (Complete) data for Cefuroxime axetil (13 total), please visit the HSDB record page.
Following oral administration, cefuroxime axetil is rapidly hydrolyzed to cefuroxime by nonspecific esterases in the intestinal mucosa and blood; the axetil moiety is metabolized to acetaldehyde and acetic acid. Cefuroxime is not metabolized and is excreted unchanged principally in urine by both glomerular filtration and tubular secretion.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 74
In neonates and children, the serum half-life of cefuroxime is inversely proportional to age.6 25 30 Following oral administration of cefuroxime axetil oral suspension in children 3 months to 12 years of age, the serum half-life of cefuroxime averages 1.4-1.9 hours.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 74
In adults, the serum or plasma half-life of cefuroxime following oral administration of commercially available cefuroxime axetil tablets or oral suspension ranges from 1.2-1.6 hours.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 74
Because cefuroxime is renally excreted, the serum half-life is prolonged in patients with reduced renal function. In a study of 20 elderly patients (mean age = 83.9 years) having a mean creatinine clearance of 34.9 mL/min, the mean serum elimination half-life was 3.5 hours.
US Natl Inst Health; DailyMed. Current Medication Information for ICEFTIN (cefuroxime axetil) powder, for suspension CEFTIN (cefuroxime axetil) tablet, film coated (July 2011). Available from, as of November 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2f439b86-59d6-4350-aeb4-c904b3781db4
Cefuroxime is usually bactericidal in action.Like other cephalosporins, the antibacterial activity of the drug results from inhibition of mucopeptide synthesis in the bacterial cell wall.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 72