

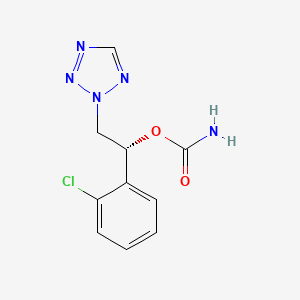

1. 2h-tetrazole-2-ethanol, Alpha-(2-chlorophenyl)-, Carbamate (ester), (alphar)-
2. Carbamic Acid (r)-(+)-1-(2-chlorophenyl)-2-(2h-tetrazol-2-yl)ethyl Ester
3. Xcopri
4. Ykp-3089
5. Ykp3089
1. Xcopri
2. 913088-80-9
3. Cenobamate [inn]
4. Ykp3089
5. Ykp-3089
6. P85x70rzws
7. [(1r)-1-(2-chlorophenyl)-2-(tetrazol-2-yl)ethyl] Carbamate
8. Carbamic Acid (r)-(+)-1-(2-chlorophenyl)-2-(2h-tetrazol-2-yl)ethyl Ester
9. Ontozry
10. Unii-p85x70rzws
11. Xcopri (tn)
12. Cenobamate [mi]
13. Ykp-3089 Cenobamate
14. Cenobamate (usan/inn)
15. Cenobamate [usan:inn]
16. Cenobamate [usan]
17. Cenobamate [who-dd]
18. Schembl1682643
19. Cenobamate [orange Book]
20. Chembl3989949
21. Gtpl10773
22. Ykp3089ykp3089
23. Dtxsid001027948
24. Ex-a3604
25. 2h-tetrazole-2-ethanol, Alpha-(2-chlorophenyl)-, 2-carbamate, (alphar)-
26. Db06119
27. 2h-tetrazole-2-ethanol, Alpha-(2-chlorophenyl)-, Carbamate (ester), (alphar)-
28. Hy-17607
29. Cs-0014686
30. D11150
31. Q27286352
32. (1r)-1-(2-chlorophenyl)-2-(tetrazol-yl)ethyl)carbamate
33. (1r)-1-(2-chlorophenyl)-2-(2h-1,2,3,4-tetrazol-2-yl)ethyl Carbamate
34. (1r)-1-(2-chlorophenyl)-2-(2h-tetrazol-2-yl)ethyl Carbamate
35. 2h-tetrazole-2-ethanol, .alpha.-(2-chlorophenyl)-, 2-carbamate, (.alpha.r)-
36. 2h-tetrazole-2-ethanol, .alpha.-(2-chlorophenyl)-, Carbamate (ester), (.alpha.r)-
37. 2h-tetrazole-2-ethanol, Alpha-(2-chlorophenyl)-, Yl)ethyl)carbamate Carbamate (ester), (.alpha.r)-
| Molecular Weight | 267.67 g/mol |
|---|---|
| Molecular Formula | C10H10ClN5O2 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 267.0523023 g/mol |
| Monoisotopic Mass | 267.0523023 g/mol |
| Topological Polar Surface Area | 95.9 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 293 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cenobamate is indicated for the treatment of partial onset seizures in adults.
Adjunctive treatment of focal-onset seizures with or without secondary generalisation in adult patients with epilepsy who have not been adequately controlled despite a history of treatment with at least 2 anti-epileptic medicinal products.
The mechanism of cenobamate is unknown, however it modulates GABAA and inhibit voltage gated sodium channels. Cenobamate is given once daily and so it has a long duration of action. The therapeutic window is wide as doses of 750mg can be well tolerated. Patients should be counselled regarding the risk of DRESS syndrome, QT interval shortening, suicidal behavior, and neurological adverse effects.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AX
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX25 - Cenobamate
Absorption
Cenobamate is 88% orally bioavailable with a Tmax of 1-4 hours. A high fat meal does not significantly impact the pharmacokinetics of cenobamate.
Route of Elimination
Cenobamate is 87.8% eliminated in the urine and 5.2% in the feces.
Volume of Distribution
The apparent volume of distribution of cenobamate is 40-50L.
Clearance
The apparent oral clearance of cenobamate is 0.45-0.63L/h for a 100-400mg/day dose.
Data regarding the metabolism of cenobamate is lacking, however it is mostly glucuronidated by UGT2B7 and UGT2B4 or oxidized by a number of cytochromes.
The terminal half life of cenobamate is 50-60h.
Cenobamate inhibits voltage gated sodium channels and is a positive GABAA modulator. However, the exact mechanism of action remains unknown. Inhibition of voltage gated sodium channels increases the threshold for generating action potentials and decreases the number of action potentials.

