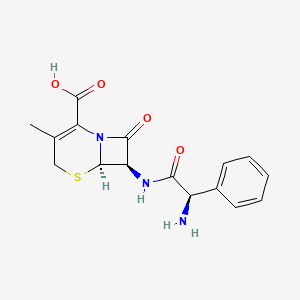

1. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-methyl-8-oxo-, (6r-(6alpha,7beta(r*)))-
2. Cefalexin
3. Cephalexin Dihydride
4. Cephalexin Hemihydrate
5. Cephalexin Hydrochloride
6. Cephalexin Monohydrate
7. Cephalexin Monohydrochloride
8. Cephalexin Monohydrochloride, Monohydrate
9. Cephalexin, (6r-(6alpha,7alpha(r*)))-isomer
10. Cephalexin, (6r-(6alpha,7beta(s*)))-isomer
11. Cephalexin, (6r-(6alpha,7beta))-isomer
12. Cephalexin, Monosodium Salt
13. Cephalexin, Monosodium Salt, (6r-(6alpha,7beta))-isomer
14. Ceporexine
15. Palitrex
1. Cefalexin
2. 15686-71-2
3. Cephacillin
4. Keflex
5. Ceporexin
6. Cepexin
7. Cephalexinum
8. Cepastar
9. Cephalexine
10. Celexin
11. Ceporex
12. Alcephin
13. Alsporin
14. Carnosporin
15. Cefablan
16. Cefaleksin
17. Cefalexina
18. Cefalexine
19. Cefalexinum
20. Durantel
21. Mamalexin
22. Sinthecillin
23. Uphalexin
24. Cefadin
25. Felexin
26. Pyassan
27. Tepaxin
28. Keflet
29. Palitrex
30. Cefalessina [dcit]
31. Cephalexin Anhydrous
32. Cefaseptin
33. Novolexin
34. Biocef
35. Keftab
36. Cefalexine [inn-french]
37. Cefalexinum [inn-latin]
38. Cefalexina [inn-spanish]
39. Panixine Disperdose
40. Lilly 66873
41. 7-(d-alpha-aminophenylacetamido)desacetoxycephalosporanic Acid
42. Ceporexine
43. Cefalexin Anhydrous
44. Cefalexin [inn]
45. Anhydrous Cephalexin
46. Ceffanex
47. Cephanasten
48. 7-beta-(d-alpha-amino-alpha-phenylacetylamino)-3-methyl-3-cephem-4-carboxylic Acid
49. Cefaloto
50. Ceforal
51. Chebi:3534
52. Cophalexin
53. Factagard
54. Kefalospes
55. Lexibiotico
56. Lopilexin
57. Neolexina
58. Ortisporina
59. Sartosona
60. Sencephalin
61. Servispor
62. Tokiolexin
63. Cefadal
64. Cefadina
65. Cefalin
66. Cefovit
67. Cephaxin
68. Erocetin
69. Ibrexin
70. Inphalex
71. Kefolan
72. Kekrinal
73. Kidolex
74. Lafarine
75. Larixin
76. Lenocef
77. Lonflex
78. Madlexin
79. Mamlexin
80. Medoxine
81. Oriphex
82. Ospexin
83. Pectril
84. Sanaxin
85. Sepexin
86. Sialexin
87. Sporicef
88. Sporidex
89. Zozarine
90. Alexin
91. Cefax
92. Cephin
93. Cepol
94. Check
95. Fexin
96. Ibilex
97. Neokef
98. Nufex
99. Oracef
100. Oroxin
101. Roceph
102. Syncl
103. Syncle
104. Synecl
105. Voxxim
106. Winlex
107. Cex
108. Cefa-iskia
109. Ceporex Forte
110. Ceporexin-e
111. Sq 20248
112. Chembl1727
113. Durantel Ds
114. L-keflex
115. (6r,7r)-7-{[(2r)-2-amino-2-phenylacetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
116. Cefalessina
117. Ed A-ceph
118. Roceph Distab
119. 5sff1w6677
120. Ncgc00159522-02
121. Ceflax
122. Dsstox_cid_2780
123. Dsstox_rid_76726
124. Dsstox_gsid_22780
125. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
126. (6r,7r)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
127. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 7-[[(2r)-aminophenylacetyl]amino]-3-methyl-8-oxo-, (6r,7r)-
128. L-cephalexin
129. Cephalexin 1-hydrate
130. Cerexin
131. Optocef
132. Cephalexin [usan:ban]
133. (6r,7r)-7-[(2r)-2-amino-2-phenylacetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
134. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-aminophenylacetyl)amino)-3-methyl-8-oxo-, (6r,7r)-
135. Smr000338536
136. S 6437
137. Cas-15686-71-2
138. Keflex (tn)
139. Hsdb 3022
140. Nsc-758162
141. Einecs 239-773-6
142. Brn 0965503
143. Taicelexin
144. 7-(d-2-amino-2-phenylacetamido)-3-methyl-delta3-cephem-4-carboxylic Acid
145. Cerexins
146. Amplex
147. Unii-5sff1w6677
148. Anhydrous Cefalexin
149. Cefalexin,(s)
150. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
151. 34632-04-7
152. Cefadros
153. Cephamasten
154. Efalexin
155. Garasin
156. Iwalexin
157. Kefloridina
158. Oracocin
159. Cefalexin (jp17)
160. Cephalexin (cefalexin)
161. Cefalexin [jan]
162. Cephalexin [mi]
163. Prestwick0_000358
164. Prestwick1_000358
165. Prestwick2_000358
166. Prestwick3_000358
167. Cephalexin [hsdb]
168. Epitope Id:117132
169. Cefalexin [who-dd]
170. Schembl2961
171. 7-(d-2-amino-2-phenylacetamido)-3-methyl-delta (sup 3)-cephem-4- Carboxylic Acid
172. Bspbio_000455
173. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure
174. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-methyl-8-oxo-, (6r-(6alpha,7beta(r*)))-
175. Mls000759527
176. Mls001424036
177. Spbio_002376
178. Bpbio1_000501
179. Gtpl4832
180. Dtxsid9022780
181. Lilly-66873
182. Bcpp000289
183. Hms2051a04
184. Hy-b0200
185. Zinc3830500
186. Tox21_111740
187. Bdbm50139896
188. Akos004119846
189. Tox21_111740_1
190. Bcp9000509
191. Ccg-100831
192. Cs-2137
193. Db00567
194. Nc00081
195. Nsc 758162
196. Ncgc00159522-03
197. Ncgc00159522-05
198. (6r,7r)-7-[[(2r)-2-amino-2-phenyl-acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
199. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(2-amino-2-phenylacetamido)-3-methyl-8-oxo-, D-
200. Ds-11971
201. B1692
202. Cefalexin, Vetranal(tm), Analytical Standard
203. C-2660
204. C06895
205. D00263
206. H10995
207. S-6437
208. Cephalexin, Antibiotic For Culture Media Use Only
209. Q411417
210. Q-200819
211. Brd-k90733503-002-03-6
212. Cefalexin, British Pharmacopoeia (bp) Reference Standard
213. Cephalexin Monohydrate, Antibiotic For Culture Media Use Only
214. Cephalexin, Pharmaceutical Secondary Standard; Certified Reference Material
215. 7-(d-.alpha.-amino-.alpha.-phenylacetamido)-3-methyl-3-cephem-4-carboxylic Acid
216. 7beta-[(2r)-2-amino-2-phenylacetamido]-3-methyl-3,4-didehydrocepham-4-carboxylic Acid
217. (6r,7r)-7-((r)-2-amino-2-phenyl-acetylamino)-3-methyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
218. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
219. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-methyl-8-oxo-,(6r-(6.alpha.,7.beta.(r*)))-
| Molecular Weight | 347.4 g/mol |
|---|---|
| Molecular Formula | C16H17N3O4S |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 347.09397721 g/mol |
| Monoisotopic Mass | 347.09397721 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 600 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Cephalexin |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cephalexin, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7- (D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4S H2O and the m... |
| Active Ingredient | Cephalexin |
| Dosage Form | Tablet; Capsule; For suspension |
| Route | Oral |
| Strength | eq 750mg base; eq 250mg base/5ml; eq 500mg base; eq 333mg base; eq 250mg base; eq 125mg base/5ml |
| Market Status | Prescription |
| Company | Ranbaxy; Sun Pharm Inds (in); Belcher Pharms; Teva; Yung Shin Pharm; Alkem Labs; Aurobindo Pharma; Lupin; Hikma Pharms; Orchid Hlthcare; Hikma |
| 2 of 4 | |
|---|---|
| Drug Name | Keflex |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Keflex Capsules (Cephalexin, USP) is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7-(D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4... |
| Active Ingredient | Cephalexin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 750mg base; eq 500mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Shionogi |
| 3 of 4 | |
|---|---|
| Drug Name | Cephalexin |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cephalexin, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7- (D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4S H2O and the m... |
| Active Ingredient | Cephalexin |
| Dosage Form | Tablet; Capsule; For suspension |
| Route | Oral |
| Strength | eq 750mg base; eq 250mg base/5ml; eq 500mg base; eq 333mg base; eq 250mg base; eq 125mg base/5ml |
| Market Status | Prescription |
| Company | Ranbaxy; Sun Pharm Inds (in); Belcher Pharms; Teva; Yung Shin Pharm; Alkem Labs; Aurobindo Pharma; Lupin; Hikma Pharms; Orchid Hlthcare; Hikma |
| 4 of 4 | |
|---|---|
| Drug Name | Keflex |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Keflex Capsules (Cephalexin, USP) is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7-(D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4... |
| Active Ingredient | Cephalexin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 750mg base; eq 500mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Shionogi |
Cephalosporins
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/CEPHALEXIN/ HAS ANTIBACTERIAL SPECTRUM SIMILAR TO THAT OF PENICILLINS... AGAINST COCCI & GRAM-POSITIVE BACILLI, PENICILLIN G IS USUALLY MORE EFFECTIVE. ...MOST PENICILLINASES DO NOT AFFECT CEPHALEXIN...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1119
...CEPHALOSPORINS ARE HIGHLY EFFECTIVE IN THERAPY OF VARIETY OF MILD-TO-SEVERE INFECTIONS DUE TO BOTH GRAM-POSITIVE & GRAM-NEGATIVE MICROORGANISMS. /CEPHALOSPORINS/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1088
...CEPHALOSPORIN IS...DRUG OF 1ST CHOICE...FOR KLEBSIELLA INFECTIONS. ... THEY ARE...VALUABLE SECONDARY AGENTS, & THEY FREQUENTLY APPEAR AS ALTERNATIVE CHOICES TO PENICILLIN. /CEPHALOSPORINS/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1091
For more Therapeutic Uses (Complete) data for CEPHALEXIN (9 total), please visit the HSDB record page.
PHYSICIAN MUST ALTER EITHER DRUG DOSAGE OR INTERVAL BETWEEN DOSES WHEN RENAL FUNCTION IS IMPAIRED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
CEPHALOSPORINS SHOULD NOT BE USED TO TREAT BACTERIAL MENINGITIS. THIS IS TRUE FOR ALL CAUSATIVE MICROORGANISMS. ...PENETRATION OF CEPHALOSPORINS INTO CSF IS POOR. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1164
INFECTIONS DUE TO ENTEROCOCCI ARE USUALLY UNAFFECTED BY THESE CMPD... ENTEROCOCCAL ENDOCARDITIS CANNOT BE CURED WITH CEPHALOSPORIN EVEN WHEN IT IS GIVEN CONCURRENTLY WITH GENTAMICIN OR STREPTOMYCIN. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
ENTEROBACTER (AEROBACTER) INFECTIONS ARE, AS A RULE, RESISTANT TO THESE CMPD. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
For more Drug Warnings (Complete) data for CEPHALEXIN (20 total), please visit the HSDB record page.
Cephalexin is indicated for the treatment of certain infections caused by susceptible bacteria. These infections include respiratory tract infections, otitis media, skin and skin structure infections, bone infections, and genitourinary tract infections.
FDA Label
Cephalexin (also called Cefalexin) is a first generation cephalosporin antibiotic. It is one of the most widely prescribed antibiotics, often used for the treatment of superficial infections that result as complications of minor wounds or lacerations. It is effective against most gram-positive bacteria through its inihibition of the cross linking reaction between N-acetyl muramicacid and N-acetylglucosamine in the cell wall, leading to cell lysis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DB - First-generation cephalosporins
J01DB01 - Cefalexin
Absorption
Well absorbed from the upper gastrointestinal tract with nearly 100% oral bioavailability. Cephalexin is not absorbed in the stomach but is absorbed in the upper intestine. Patients taking 250mg of cephalexin reach a maximum plasma concentration of 7.7mcg/mL and patients taking 500mg reach 12.3mcg/mL.
Route of Elimination
Cephalexin is over 90% excreted in the urine after 6 hours by glomerular filtration and tubular secretion with a mean urinary recovery of 99.3%. Cephalexin is unchanged in the urine.
Volume of Distribution
5.2-5.8L.
Clearance
Clearance from one subject was 376mL/min.
LESS THAN 10 TO 15%...IS BOUND TO PLASMA PROTEIN, & PLASMA DRUG CONCN FALL RAPIDLY... MORE THAN 90%...IS EXCRETED UNALTERED IN URINE WITHIN 6 HR, PRIMARILY BY RENAL TUBULAR SECRETION. ...THERAPEUTICALLY EFFECTIVE CONCN ARE STILL ACHIEVED IN URINE OF PT WITH DECR RENAL FUNCTION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
CEPHALEXIN...IS WELL ABSORBED FROM GI TRACT. PEAK PLASMA CONCN, REACHED @ ABOUT 1 HR AFTER INGESTION OF DRUG, ARE APPROX 9 & 18 UG/ML AFTER ORAL DOSES OF 250 & 500 MG, RESPECTIVELY. INGESTION OF FOOD MAY DELAY ABSORPTION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
CEPHALEXIN IS ALSO EXCRETED INTO BILE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
BOTH ABSORPTION & EXCRETION OF CEPHALEXIN ARE IMPAIRED IN NEW-BORN INFANTS, WHERE 24-HR URINARY RECOVERY OF ANTIBIOTIC ACCOUNTED FOR 5-66% OF DAILY ORAL DOSE.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 177
For more Absorption, Distribution and Excretion (Complete) data for CEPHALEXIN (14 total), please visit the HSDB record page.
Cephalexin is not metabolized in the body.
The half life of cephalexin is 49.5 minutes in a fasted state and 76.5 minutes with food though these times were not significantly different in the study.
LESS THAN 10 TO 15%...IS BOUND TO PLASMA PROTEIN, & PLASMA DRUG CONCN FALL RAPIDLY, T/2 OF CEPHALEXIN NORMALLY BEING ABOUT 40 MIN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
/IN RATS/ RATIOS OF BONE TO SERUM CONCN AVG...1:9 FOR CEPHALEXIN DURING 0.25-4 HR AFTER /ORAL/ DOSING. DESPITE DIFFERENCES IN CONCN; T/2 IN BONE & SERUM WERE SIMILAR.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 452
PEAK TIME, T/2 OF ELIMINATION, T/2 OF ABSORPTION, & VOL OF DISTRIBUTION WERE ALL SIMILAR FOLOWING ADMIN OF EITHER 1 OR 2 G OF CEPHALEXIN.
PMID:438352 CHOW M ET AL; J CLIN PHARMACOL 19 (4): 185-94 (1979)
The serum half-life of cephalexin is 0.5-1.2 hr in adults with normal renal function. The serum half-life of the drug is reported to be about 5 hr in neonates and 2.5 hr in children 3-12 mo of age. In one study, the serum half-life was 7.7 hr in adults with creatinine clearances of 9.2 ml/min and 13.9 hr in adults with creatinine clearances of 4 ml/min.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 166
Cephalexin is a first generation cephalosporin antibiotic. Cephalosporins contain a beta lactam and dihydrothiazide. Unlike penicillins, cephalosprins are more resistant to the action of beta lactamase. Cephalexin inhibits bacterial cell wall synthesis, leading breakdown and eventualy cell death.
CEPHALOTHIN & ITS CONGENERS INHIBIT BACTERIAL CELL-WALL SYNTHESIS IN MANNER SIMILAR TO THAT OF PENICILLIN. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
The penicillins and their metabolites are potent immunogens because of their ability to combine with proteins and act as haptens for acute antibody-mediated reactions. The most frequent (about 95 percent) or "major" determinant of penicillin allergy is the penicilloyl determinant produced by opening the beta-lactam ring of the penicillin. This allows linkage of the penicillin to protein at the amide group. "Minor" determinants (less frequent) are the other metabolites formed, including native penicillin and penicilloic acids. /Penicillins/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 953
Bactericidal; action depends on ability to reach and bind penicillin-binding proteins located in bacterial cytoplasmic membranes; cephalosporins inhibit bacterial septum and cell wall synthesis, probably by acylation of membrane-bound transpeptidase enzymes. This prevents cross-linkage of peptidoglycan chains, which is necessary for bacterial cell wall strength and rigidity. Also, cell division and growth are inhibited, and lysis and elongation of susceptible bacteria frequently occur. Rapidly dividing bacteria are those most susceptible to the action of cephalosporins. /Cephalosporins/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 679
CEPHALOSPORIN C IS VERY RESISTANT TO ACTION OF PENICILLINASE, FOR WHICH IT IS BOTH COMPETITIVE & NONCOMPETITIVE INHIBITOR, DEPENDING ON SUBSTRATE TESTED... /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160