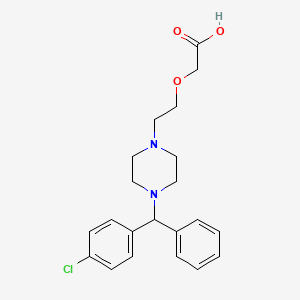

1. (2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)acetic Acid
2. Alerlisin
3. Aller-tec
4. Cetalerg
5. Ceterifug
6. Ceti Tad
7. Ceti-puren
8. Cetiderm
9. Cetidura
10. Cetil Von Ct
11. Cetilich
12. Cetirigamma
13. Cetirizin Al
14. Cetirizin Azu
15. Cetirizin Basics
16. Cetirizine Dihydrochloride
17. Cetirlan
18. Dihydrochloride, Cetirizine
19. P 071
20. P-071
21. P071
22. Reactine
23. Virlix
24. Voltric
25. Zetir
26. Zirtek
27. Zyrtec
1. 83881-51-0
2. Cetirizina
3. Cetirizin
4. Virlix
5. Cetirizinum
6. Zirtek
7. Cetiderm
8. 2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)acetic Acid
9. Cetirizine Hydrochloride
10. Ac-170
11. Cetirizine (inn)
12. 2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]acetic Acid
13. Cetryn
14. Setir
15. Ziptek
16. Chembl1000
17. Chebi:3561
18. 1133210-23-7
19. Cetirizinum [latin]
20. (r)-cetirizine (dihydrochloride)
21. Hitrizin Film Tablet
22. Cetirizina [spanish]
23. Yo7261me24
24. (2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)acetic Acid
25. (2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)acetic Acid
26. 2-[2-[4-[(4-chlorophenyl)-phenyl-methyl]piperazin-1-yl]ethoxy]acetic Acid Dihydrichloride
27. P071
28. Zyrlex
29. Cetirizine [inn]
30. 2-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)ethoxy)acetic Acid
31. Acetic Acid, [2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]-
32. Cetirizine [inn:ban]
33. Xarlin
34. Acetic Acid, (2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)-
35. Cetiderm (tn)
36. Levocetirizine (dihydrochloride)
37. Irizine
38. Benaday
39. Humex
40. Unii-yo7261me24
41. Cetrizine Dihcl
42. [2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic Acid
43. Hsdb 7739
44. 2-[2-[4-[(r)-(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]acetic Acid
45. Cetirizine [mi]
46. Prestwick0_000503
47. Prestwick1_000503
48. Prestwick2_000503
49. Prestwick3_000503
50. Cetirizine [hsdb]
51. Cetirizine [vandf]
52. Schembl4176
53. Timtec1_002075
54. Cetirizine [who-dd]
55. Bspbio_000425
56. Bspbio_002302
57. Spbio_002346
58. Bpbio1_000469
59. Gtpl1222
60. Dtxsid4022787
61. Bdbm22890
62. Hms1539o07
63. Hms3259d06
64. Hms3370i19
65. Hms3393n10
66. Amy22576
67. Bcp08787
68. Bcp28477
69. Bbl009968
70. Cet83881-51-0
71. Stk711108
72. (r)-cetirizine-[d4] Dihydrochloride
73. [(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethyl)oxy]acetic Acid
74. 2-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]acetic Acid
75. Acetic Acid, 2-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]-
76. Akos003589059
77. Akos021729457
78. Ac-5545
79. Cs-0886
80. Db00341
81. Nc00696
82. Sdccgsbi-0206838.p002
83. Ncgc00167781-01
84. Ncgc00167781-02
85. Ncgc00167781-03
86. Ncgc00167781-04
87. Ncgc00167781-07
88. Ncgc00167781-12
89. Ncgc00167781-18
90. As-77105
91. Hy-17042
92. Sbi-0206838.p001
93. Db-049948
94. Ft-0603001
95. Ft-0664488
96. En300-52521
97. 81c510
98. C07778
99. D07662
100. E75781
101. L000655
102. Q423075
103. J-005737
104. Brd-a42571354-300-05-4
105. Brd-a42571354-300-07-0
106. Z1182332250
107. [2-[4-[(4-chlorophenyl)-phenyl Methyl]-1-piperazinyl] Ethoxy] Acetic Acid
108. [2-[4-[(4-chlorophenyl)phenylmethyl] 1-piperazinyl]-ethoxy] Acetic Acid
109. [2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]-ethoxy]acetic Acid
110. 2-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)ethoxy)aceticacid
111. 2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazino}ethoxy)acetic Acid
112. (+/-)-(2-(4-(p-chloro-a-phenylbenzyl)-1-piperazinyl)ethoxy)acetic Acid
113. Acetic Acid, (2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)-, (+/-)-
| Molecular Weight | 388.9 g/mol |
|---|---|
| Molecular Formula | C21H25ClN2O3 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 388.1553704 g/mol |
| Monoisotopic Mass | 388.1553704 g/mol |
| Topological Polar Surface Area | 53 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 443 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Zyrtec |
| PubMed Health | Ketotifen |
| Drug Classes | Mast Cell Stabilizer, Ophthalmologic Agent, Piperidine |
| Active Ingredient | Cetirizine hydrochloride |
| Dosage Form | Syrup |
| Route | Oral |
| Strength | 5mg/5ml |
| Market Status | Prescription |
| Company | Mcneil Consumer |
| 2 of 2 | |
|---|---|
| Drug Name | Zyrtec |
| PubMed Health | Ketotifen |
| Drug Classes | Mast Cell Stabilizer, Ophthalmologic Agent, Piperidine |
| Active Ingredient | Cetirizine hydrochloride |
| Dosage Form | Syrup |
| Route | Oral |
| Strength | 5mg/5ml |
| Market Status | Prescription |
| Company | Mcneil Consumer |
Cetirizine alone or in fixed combination with pseudoephedrine hydrochloride is used for self-medication to provide symptomatic relief of seasonal allergic rhinitis (e.g., hay fever) or other upper respiratory allergies. Cetirizine alone or in fixed combination with pseudoephedrine hydrochloride also is used for the symptomatic treatment of perennial allergic rhinitis. It is recommended that the fixed combination generally be used only when both the antihistamine and nasal decongestant activity of the combination preparation are needed concurrently. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 26
Cetirizine is used for self-medication to provide symptomatic relief of pruritus associated with chronic idiopathic urticaria (e.g., hives). /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 26
MEDICATION (VET): The efficacy of antihistamines for the treatment of pruritus is highly variable. The most commonly used antihistamines include ... cetirizine hydrochloride ... . /Cetirizine hydrochloride/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 677
In a controlled study of 1 week's duration in patients 6-11 months of age, those receiving cetirizine exhibited greater irritability/fussiness than those receiving placebo. In a controlled study in patients 12 months of age and older, insomnia occurred more frequently with cetirizine than with placebo (9 vs 5.3%, respectively). In those who received 5 mg or more daily, fatigue occurred in 3.6 or 1.3% and malaise in 3.5 or 1.8% of those receiving cetirizine or placebo, respectively.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 27-8
Fatigue or dizziness occurred in 5.9 or 2%, respectively, of patients 12 years of age and older receiving cetirizine, whereas these effects occurred in 2.6 or 1.2%, respectively, of patients receiving placebo. Headache was reported in more than 2% of patients 12 years of age and older receiving the drug; however, headache occurred more frequently in patients receiving placebo. In clinical trials in patients 6-11 years of age, headache occurred in 11, 14, or 12.3% of patients receiving 5-mg doses, 10-mg doses, or placebo, respectively. Abnormal coordination, ataxia, confusion, abnormal thinking, agitation, amnesia, anxiety, depersonalization, depression, emotional lability, euphoria, impaired concentration, insomnia, sleep disorders, nervousness, paroniria, dysphonia, asthenia, malaise, pain, hyperesthesia, hypoesthesia, hyperkinesia, hypertonia, migraine headache, myelitis, paralysis, paresthesia, ptosis, syncope, tremor, twitching, and vertigo have been reported in less than 2% of patients 12 years of age and older and children 6-11 years of age receiving cetirizine hydrochloride; however, a causal relationship to the drug has not been established. Aggressive reaction, seizures, hallucinations, suicidal ideation, and suicide have been reported rarely during postmarketing surveillance.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 27
The most frequent adverse effect in patients 12 years of age and older reported during cetirizine therapy is somnolence, occurring in 11, 14, or 6% of patients receiving 5-mg doses, 10-mg doses, or placebo, respectively. Overall, somnolence has been reported in 13.7 or 6.3% of patients receiving cetirizine or placebo, respectively. In addition, in clinical trials in patients 6-11 years of age, somnolence occurred in 1.9, 4.2, or 1.3% of patients receiving 5-mg doses, 10-mg doses, or placebo, respectively. Discontinuance of therapy because of somnolence has been reported in 1 or 0.6% of patients receiving cetirizine or placebo, respectively.1 3 In patients 6-24 months of age, somnolence occurred with essentially the same frequency in those who received cetirizine versus placebo.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 27
Adverse effects reported in 1% or more of patients 12 years of age and older with seasonal allergic rhinitis who received extended-release tablets of cetirizine hydrochloride in fixed combination with pseudoephedrine hydrochloride (Zyrtec-D) included insomnia, dry mouth, fatigue, somnolence, pharyngitis, epistaxis, accidental injury, dizziness, and sinusitis.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 27
For more Drug Warnings (Complete) data for Cetirizine (31 total), please visit the HSDB record page.
**Seasonal Allergic Rhinitis**: Indicated for the relief of symptoms associated with seasonal allergic rhinitis caused by allergens such as ragweed, grass and tree pollens in adults and children 2 years of age and above. Symptoms treated effectively include sneezing, rhinorrhea, nasal pruritus, ocular pruritus, tearing, and redness of the eyes. **Perennial allergic rhinitis**: This drug is indicated for the relief of symptoms associated with perennial allergic rhinitis due to allergens including dust mites, animal dander, and molds in adults and children 6 months of age and older. Symptoms treated effectively include sneezing, rhinorrhea, postnasal discharge, nasal pruritus, ocular pruritus, and tearing. **Chronic urticaria**: Cetirizine is indicated for the treatment of the uncomplicated skin manifestations of chronic idiopathic urticaria in adults and children 6 months of age and older. It markedly reduces the occurrence, severity, and duration of hives and significantly reduces pruritus.
FDA Label
**General effects and respiratory effects** Cetirizine, the active metabolite of the piperazine H1-receptor antagonist hydroxyzine, minimizes or eliminates the symptoms of chronic idiopathic urticaria, perennial allergic rhinitis, seasonal allergic rhinitis, allergic asthma, physical urticaria, and atopic dermatitis. The clinical efficacy of cetirizine for allergic respiratory diseases has been well established in numerous trials. **Effects on urticaria/anti-inflammatory effects** It has anti-inflammatory properties that may play a role in asthma management. There is evidence that cetirizine improves symptoms of urticaria. Marked clinical inhibition of a wheal and flare response occurs in infants, children as well as adults within 20 minutes of one oral dose and lasts for 24 h. Concomitant use of cetirizine reduces the duration and dose of topical anti-inflammatory formulas used for the treatment of atopic dermatitis.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Histamine H1 Antagonists, Non-Sedating
A class of non-sedating drugs that bind to but do not activate histamine receptors (DRUG INVERSE AGONISM), thereby blocking the actions of histamine or histamine agonists. These antihistamines represent a heterogenous group of compounds with differing chemical structures, adverse effects, distribution, and metabolism. Compared to the early (first generation) antihistamines, these non-sedating antihistamines have greater receptor specificity, lower penetration of BLOOD-BRAIN BARRIER, and are less likely to cause drowsiness or psychomotor impairment. (See all compounds classified as Histamine H1 Antagonists, Non-Sedating.)
R06AE07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R06 - Antihistamines for systemic use
R06A - Antihistamines for systemic use
R06AE - Piperazine derivatives
R06AE07 - Cetirizine
S - Sensory organs
S01 - Ophthalmologicals
S01G - Decongestants and antiallergics
S01GX - Other antiallergics
S01GX12 - Cetirizine
Absorption
Cetirizine was rapidly absorbed with a time to maximum concentration (Tmax) of about 1 hour after oral administration of tablets or syrup formulation in adult volunteers. Bioavailability was found to be similar between the tablet and syrup dosage forms. When healthy study volunteers were given several doses of cetirizine (10 mg tablets once daily for 10 days), a mean peak plasma concentration (Cmax) of 311 ng/mL was measured. **Effect of food on absorption** Food had no effect on cetirizine exposure (AUC), however, Tmax was delayed by 1.7 hours and Cmax was decreased by 23% in the fed state.
Route of Elimination
Mainly eliminated in the urine,. Between 70 85% of an orally administered dose can be found in the urine and 10 13% in the feces.
Volume of Distribution
Apparent volume of distribution: 0.44 +/- 0.19 L/kg.
Clearance
Apparent total body clearance: approximately 53 mL/min. Cetirizine is mainly eliminated by the kidneys,. Dose adjustment is required for patients with moderate to severe renal impairment and in patients on hemodialysis.
A mass balance study in 6 healthy male volunteers indicated that 70% of the administered radioactivity was recovered in the urine and 10% in the feces. Approximately 50% of the radioactivity was identified in the urine as unchanged drug. Most of the rapid increase in peak plasma radioactivity was associated with parent drug, suggesting a low degree of first-pass metabolism. Cetirizine is metabolized to a limited extent by oxidative O-dealkylation to a metabolite with negligible antihistaminic activity. The enzyme or enzymes responsible for this metabolism have not been identified.
US Natl Inst Health; DailyMed. Current Medication Information for Zyrtec - cetirizine hydrochloride (May 2006). Available from, as of July 2, 2009 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2115
The mean plasma protein binding of cetirizine is 93%, independent of concentration in the range of 25-1000 ng/mL, which includes the therapeutic plasma levels observed.
US Natl Inst Health; DailyMed. Current Medication Information for Zyrtec - cetirizine hydrochloride (May 2006). Available from, as of July 2, 2009 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2115
Cetirizine was rapidly absorbed with a time to maximum concentration (Tmax) of approximately 1 hour following oral administration of tablets, chewable tablets or syrup in adults. Comparable bioavailability was found between the tablet and syrup dosage forms. Comparable bioavailability was also found between the Zyrtec tablet and the Zyrtec chewable tablet taken with or without water. When healthy volunteers were administered multiple doses of cetirizine (10 mg tablets once daily for 10 days), a mean peak plasma concentration (Cmax) of 311 ng/mL was observed. No accumulation was observed. Cetirizine pharmacokinetics were linear for oral doses ranging from 5 to 60 mg. Food had no effect on the extent of exposure (AUC) of the cetirizine tablet or chewable tablet, but Tmax was delayed by 1.7 hours and 2.8 hours respectively, and Cmax was decreased by 23% and 37%, respectively in the presence of food.
US Natl Inst Health; DailyMed. Current Medication Information for Zyrtec - cetirizine hydrochloride (May 2006). Available from, as of July 2, 2009 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2115
When pediatric patients aged 7 to 12 years received a single, 5-mg oral cetirizine capsule, the mean Cmax was 275 ng/mL. Based on cross-study comparisons, the weight-normalized, apparent total body clearance was 33% greater and the elimination half-life was 33% shorter in this pediatric population than in adults. In pediatric patients aged 2 to 5 years who received 5 mg of cetirizine, the mean Cmax was 660 ng/mL. Based on cross-study comparisons, the weight-normalized apparent total body clearance was 81 to 111% greater and the elimination half-life was 33 to 41% shorter in this pediatric population than in adults. In pediatric patients aged 6 to 23 months who received a single dose of 0.25 mg/kg cetirizine oral solution (mean dose 2.3 mg), the mean Cmax was 390 ng/mL. Based on cross-study comparisons, the weight-normalized, apparent total body clearance was 304% greater and the elimination half-life was 63% shorter in this pediatric population compared to adults. The average AUC(0-t) in children 6 months to <2 years of age receiving the maximum dose of cetirizine solution (2.5 mg twice a day) is expected to be two-fold higher than that observed in adults receiving a dose of 10 mg cetirizine tablets once a day.
US Natl Inst Health; DailyMed. Current Medication Information for Zyrtec - cetirizine hydrochloride (May 2006). Available from, as of July 2, 2009 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2115
For more Absorption, Distribution and Excretion (Complete) data for Cetirizine (7 total), please visit the HSDB record page.
A mass balance clinical trial comprised of 6 healthy male study volunteers showed that 70% of the administered radioactivity was measured in the urine and 10% in the feces after cetirizine administration. About 50% of the radioactivity was measured in the urine as unchanged cetirizine. Most of the rapid increase in peak plasma radioactivity was related to the parent drug, implying a low level of first pass metabolism. This prevents potential interactions of cetirizine with drugs interacting with hepatic cytochrome enzymes. Cetirizine is metabolized partially by oxidative O-dealkylation to a metabolite with insignificant antihistaminic activity. The enzyme or enzymes responsible for this step in cetirizine metabolism have not yet been identified.
A mass balance study in 6 healthy male volunteers indicated that 70% of the administered radioactivity was recovered in the urine and 10% in the feces. Approximately 50% of the radioactivity was identified in the urine as unchanged drug. Most of the rapid increase in peak plasma radioactivity was associated with parent drug, suggesting a low degree of first-pass metabolism. Cetirizine is metabolized to a limited extent by oxidative O-dealkylation to a metabolite with negligible antihistaminic activity. The enzyme or enzymes responsible for this metabolism have not been identified.
US Natl Inst Health; DailyMed. Current Medication Information for Zyrtec - cetirizine hydrochloride (May 2006). Available from, as of July 2, 2009 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2115
Pharmacokinetic parameters of hydroxyzine and its active metabolite cetirizine were determined after oral and intravenous administration of 2 mg kg(-1) of hydroxyzine to six healthy dogs. Plasma drug levels were determined with high-pressure liquid chromatography. Pharmacodynamic studies evaluated the suppressive effect on histamine and anticanine IgE-mediated cutaneous wheal formation. Pharmacokinetic and pharmacodynamic correlations were determined with computer modelling. The mean systemic availability of oral hydroxyzine was 72%. Hydroxyzine was rapidly converted to cetirizine regardless of the route of administration. The mean area-under-the-curve was eight and ten times higher for cetirizine than hydroxyzine after intravenous and oral dosing, respectively. After oral administration of hydroxyzine, the mean peak concentration of cetirizine was approximately 2.2 microg mL(-1) and that of hydroxyzine 0.16 ug mL(-1). The terminal half-life for cetirizine varied between 10 and 11 hr after intravenous and oral administration of hydroxyzine. A sigmoidal relationship was fit to the data comparing cetirizine plasma concentration to wheal suppression. Maximum inhibition (82% and 69% for histamine and anticanine IgE-mediated skin reactions, respectively) was observed during the first 8 hr, which correlated with a plasma concentration of cetirizine greater than 1.5 ug mL(-1). Pharmacological modelling suggested that increasing either hydroxyzine dosages or frequencies of administration would not result in histamine inhibition superior to that obtained with twice daily hydroxyzine at 2 mg kg(-1). In conclusion, there was rapid conversion of hydroxyzine to cetirizine. The reduction of wheal formation appeared almost entirely due to cetirizine. Pharmacodynamic modelling predicted that maximal antihistamine effect would occur with twice daily oral administration of hydroxyzine at 2 mg kg(-1).
PMID:18980631 Bizikova P et al; Vet Dermatol 19 (6): 348-57 (2008).
Plasma elimination half-life is 8.3 hours.
The mean elimination half-life in 146 healthy volunteers across multiple pharmacokinetic studies was 8.3 hours ... .
US Natl Inst Health; DailyMed. Current Medication Information for Zyrtec - cetirizine hydrochloride (May 2006). Available from, as of July 2, 2009 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2115
The pharmacokinetics of the second generation H1-receptor antagonist cetirizine were studied in 15 infants and toddlers (mean +/- SD age, 12.3 +/- 5.4 months) who were treated with a single 0.25 mg/kg dose of cetirizine solution. ... The elimination half-life was 3.1 +/- 1.8 hours ...
PMID:9084458 Spicak V et al; Clin Pharmacol Ther 61 (3): 325-30 (1997).
Cetirizine, a metabolite of _hydroxyzine_, is an antihistamine drug. Its main effects are achieved through selective inhibition of peripheral H1 receptors. The antihistamine activity of cetirizine has been shown in a variety of animal and human models. _In vivo_ and _ex vivo_ animal models have shown insignificant anticholinergic and antiserotonergic effects. In clinical studies, however, dry mouth was found to be more frequent with cetirizine than with a placebo. In vitro receptor binding studies have demonstrated no detectable affinity of cetirizine for histamine receptors other than the H1 receptors. Studies with radiolabeled cetirizine administration in the rat have demonstrated insignificant penetration into the brain. _Ex vivo_ studies in the mouse have shown that systemically administered cetirizine does not occupy cerebral H1 receptors significantly.
Cetirizine, a human metabolite of hydroxyzine, is an antihistamine; its principal effects are mediated via selective inhibition of peripheral H1 receptors. The antihistaminic activity of cetirizine has been clearly documented in a variety of animal and human models. In vivo and ex vivo animal models have shown negligible anticholinergic and antiserotonergic activity. In clinical studies, however, dry mouth was more common with cetirizine than with placebo. In vitro receptor binding studies have shown no measurable affinity for other than H1 receptors. Autoradiographic studies with radiolabeled cetirizine in the rat have shown negligible penetration into the brain. Ex vivo experiments in the mouse have shown that systemically administered cetirizine does not significantly occupy cerebral H1 receptors.
US Natl Inst Health; DailyMed. Current Medication Information for Zyrtec - cetirizine hydrochloride (May 2006). Available from, as of July 2, 2009 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2115